Martin Fussenegger, Ph.D.
AIMBE College of Fellows Class of 2008 For pioneering contributions to cell culture engineering and molecular methods for diagnosis and therapy of disease at the genetic level.
Electrogenetics Study Finds We Could One Day Control Our Genes With Wearables
Via Singularity Hub | August 4, 2023The components sound like the aftermath of a shopping and spa retreat: three AA batteries. Two electrical acupuncture needles. One plastic holder that’s usually attached to battery-powered fairy lights. But together they merge into a powerful stimulation device that uses household batteries to control gene expression in cells.
The idea seems wild, but a new study in Nature Metabolism this week showed that it’s possible. The team, led by Dr. Martin Fussenegger at ETH Zurich and the University of Basel in Switzerland, developed a system that uses direct-current electricity—in the form of batteries or portable battery banks—to turn on a gene in human cells in mice with a literal flip of a switch… Continue reading.
Biomedical Tattoo Detects Cancer-Associated Hypercalcemia
Via Hospimedica | May 1, 2018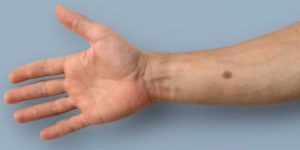
Image: A new study explains how increased calcium levels trigger the production of melanin, causing a mole to form (Photo courtesy of ETH Zurich).
A new study describes how a mole-like synthetic implant recognizes the elevated levels of calcium in the blood associated with incipient prostate, lung, colon, and breast cancer tumors.
Developed by researchers at ETH Zurich (Switzerland) and the University of Basel (Switzerland), the so-called early warning system is comprised of an encapsulated gene network integrated into human body cells implanted under the skin, where it constantly monitors blood calcium levels. When calcium levels exceed a pre-determined threshold, a synthetic signaling cascade expresses transgenic tyrosinase, which triggers the production of melanin in the genetically modified cells. The skin then forms a brown mole that is visible to the naked eye.
The researchers validated the design in wild-type mice inoculated with hypercalcemic breast and colon adenocarcinoma cells. The mice, which were implanted with the subcutaneous encapsulated engineered cells, all developed tattoos, whereas no tattoos were seen in animals inoculated with normo-calcemic tumor cells. The study also confirmed that the synthetic biomedical mole could not only be detected with the naked eye, but could also optically quantified. All animals remained asymptomatic throughout the 38-day experimental period. The study was published on April 18, 2018, in Science Translational Medicine… Continue reading.
Novel Pain Management Approach Uses Spearmint to Trigger Pain Relief
Via MD Magazine | February 15, 2018Researchers have developed a novel strategy that could usher in a new era of pain management and combat the persistent problem of painkiller addiction.
A new study highlighted a bioengineering-based method that involves engineering cells that can target pain receptors and then tie those cells to an aroma-based triggering mechanism. “Our proof-of-principle findings indicate that therapies based on engineered cells can achieve robust, tunable and on-demand analgesia for the long-term management of chronic pain,” the study authors wrote.
The strategy works in a number of steps. First, researchers created “microencapsulated human designer cells” that produce Huwentoxin-IV, a potent analgesic peptide that selectively shuts down a pain-triggering sodium channel called Nav1.7. Next, they rewired an olfactory receptor, OR1A1, so that it induced the expression of a variant of Huwentoxin-IV. The researchers chose an olfactory receptor that is responsive to R-carvone, a chemical that smells like spearmint.
Thus, the researchers created a mechanism in which the smell of spearmint leads to the production of Huwentoxin-IV, which in turn eliminates pain. The so-called “AromaCells” were tested in mouse models, and the results suggested the mice achieved significant and lasting pain relief with exposed to spearmint.
Martin Fussenegger, PhD, the lead author and a professor in the Department of Biosystems Science and Bioengineering at ETH Zurich (Swiss Federal Institute of Technology, Zurich), said the system circumvents one of the key problems associated with pain management… Continue reading.
Controlling Genes With Your Thoughts
Via Phys.org | November 11, 2014Researchers led by ETH Zurich professor Martin Fussenegger have constructed the first gene network that can be controlled by our thoughts. The inspiration for this development was a game that picks up brainwaves in order to guide a ball through an obstacle course.
It sounds like something from the scene in Star Wars where Master Yoda instructs the young Luke Skywalker to use the force to release his stricken X-Wing from the swamp: Marc Folcher and other researchers from the group led by Martin Fussenegger, Professor of Biotechnology and Bioengineering at the Department of Biosystems (D-BSSE) in Basel, have developed a novel gene regulation method that enables thought-specific brainwaves to control the conversion of genes into proteins – called gene expression in technical terms.
"For the first time, we have been able to tap into human brainwaves, transfer them wirelessly to a gene network and regulate the expression of a gene depending on the type of thought. Being able to control gene expression via the power of thought is a dream that we’ve been chasing for over a decade," says Fussenegger.
 AIMBE
AIMBE
