Among AIMBE’s most important roles is the promotion of public policies that foster continued advancement in medical and biological engineering (MBE). We educate and influence public officials, regulators, the media and general public about the positive impact MBE has on virtually every sector of society – from human health to a vibrant economy. AIMBE advocates for legislative, monetary and regulatory solutions that assistant our engineering community at each stage of the innovation ecosystem – from the research lab to the bedside of a patient.
How We Advocate
AIMBE’s Coalition Partnerships
AIMBE partners with like-minded organizations to advance policies that support medical and biological engineering innovation. As a member of several coalitions, AIMBE works alongside industry, academic, and professional groups to advocate for sustained federal research funding, regulatory improvements, and policies that promote scientific advancement. Through these partnerships, AIMBE strengthens its voice in Washington, ensuring that the interests of the biomedical engineering community are well represented.
AIMBE is a proud partner in:
- Ad Hoc Group for Medical Research (led by AAMC)- Coalition of nearly 400 patient groups, medical and scientific societies, academic and research organizations, and industry
- Coalition for National Science Funding- An alliance of over 140 professional organizations, universities, and businesses
- ResearchAmerica!- Over 300 members institutions spanning the research spectrum from bench to bedside including academia, industry, professional societies, health groups
- American Institute of Physics- Representing 32 organizations across the spectrum of the physical sciences
AIMBE’s Commitment to Advocacy Through Coalition Engagement
As part of its coalition work, AIMBE joins sign-on letters that amplify the collective voice of the scientific and engineering community on critical issues affecting research funding, innovation, and public health.
See recent community letters that AIMBE has signed:
- Research!America Letter on FY25 Funding
- Union of Concerned Scientists Letter on Protecting the Future of Science
- American Institute of Biological Sciences Letter on Protecting U.S. Scientific Research and Integrity
- Ad Hoc Group for Medical Research Letter on FY26 Funding
- Community Letter on FY26 ARPA-H Funding
- American Astronomical Society Letter to Congress Regarding National Science Foundation Reorganizations and Cuts
IMPACTING POLICIES AFFECTING INDUSTRY
AIMBE Leads Stakeholder Briefings on PFAS in Medical Devices
AIMBE’s Industry Council, chaired by Dr. SuPing Lyu, is leading the charge to highlight the importance of Per- and Polyfluorinated Substances (PFAS) to the medical device industry. These so-called “forever chemicals” are rightfully being scrutinized to protect our health and environment; however, a very small number of these chemicals play critical roles in making sure that our medical devices save tens of millions of patients’ lives. AIMBE hosted a roundtable meeting to gather stakeholders to discuss the use of PFAS in medical devices and how they may be impacted by supply and pending regulatory changes. Perspectives including suppliers/processors of PFAS, medical device companies (users of PFAS), federal agencies (e.g., FDA, NIH, NIST, EPA, etc.), academia, public health, policy, and more were represented. A congressional lunch briefing, hosted by AIMBE, was held on the same topic to educate lawmakers and staffers about the importance of fluorinated polymers to live-saving medical devices. The briefing featured AIMBE Fellows Dr. Kim Chaffin, Medtronic and Dr. William Wagner, University of Pittsburgh.
At the state level, AIMBE Fellows, Dr. Nadine Ding (Abbott) and Dr. Bill Wagner (University of Pittsburgh), presented to the Maine State Chamber of Commerce on the importance of PFAS in medical devices in the context of Maine’s PFAS in Products Law which mandated that manufacturers of products containing intentionally added PFAS disclose their presence to the Department of Environmental Protection (DEP) by January 1, 2023 (later extended to 2025). It also prohibited the sale of such products if they failed to report and set a ban on all products containing intentionally added PFAS starting in 2030, with few exceptions. Drs. Ding and Wagner discussed the impact this law would have on medical devices and patients. Following their impactful comments, on April 16, 2024, Maine Governor Janet Mills approved significant revisions to the PFAS in Products Law. The amendments eliminate the reporting obligation unless the DEP determines that the use of PFAS in the product is deemed “currently unavoidable.” They also remove the deadline for such notification and establish exemptions from the law for specific products containing intentionally added PFAS. Importantly, medical devices were included on the list of exemptions. Click here for more information.



AIMBE Lends Expertise to the FDA (Network of Experts Agreement)
AIMBE was one of the first organizations to partner with the Center for Devices and Radiological Health (CDRH) at the Food and Drug Administration (FDA) to sign a Network of Experts Agreement.
The CDRH is responsible for regulating firms who manufacture, repackage, relabel, and/or import medical devices sold in the United States. In order to better meet this responsibility, the CDRH identified a need for additional medical and engineering expertise from the broader scientific and engineering community to enhance the knowledge base of their own staff. Through the Network of Experts Agreement, AIMBE’s College of Fellows—around 1000 innovators and pioneers from the medical and biological engineering community—will be called to provide scientific and engineering knowledge to the CDRH at the FDA’s discretion. This will provide the FDA with access to leading engineers in new and emerging technologies in bioengineering to better fulfill their mission.
By partnering with the CDRH, AIMBE is committing to providing access to the College of Fellows whenever an expert is needed. Through this collaboration, AIMBE continues to demonstrate its role as a major thought-leader in medical and biological engineering.
Jeffrey Shuren, M.D., J.D., director of the Center for Devices and Radiological released this statement: “Rapid access to the expertise of AIMBE’s distinguished College of Fellows will help to broaden CDRH’s scientific viewpoints and assure that we consider the most current information on emerging fields of science and pioneering biomedical technologies related to medical devices.”
AIMBE is proud to be providing access to our College of Fellows, comprised of the most cutting-edge and pioneering leaders of biomedical engineering, to the CDRH at the FDA. Our mission includes building strong relationships with government agencies in order to advance the potential for medical and biological engineering to improve our quality of life, and this Network of Experts Agreement contributes greatly to that goal. We consider this a new opportunity for public service leveraging the considerable scientific and medical knowledge of AIMBE Fellows to benefit all Americans.
MAKING THE CASE FOR FEDERAL INVESTMENTS IN BIOMEDICAL INNOVATION
Advocating for AIMBE Fellows with NIH Leadership
AIMBE has undertaken a comprehensive project tracking NIH funding for AIMBE Fellows to represent the interests of Fellows with leadership at the federal agency, ensuring AIMBE serves as your advocate at NIH.
AIMBE Hosts Regional Advocacy Event in Boston
AIMBE hosted an inaugural regional advocacy event with Boston University, showcasing the innovative medtech advancements of AIMBE Fellows from BU, Harvard, MIT, Northeastern, and Tufts. The event featured hands-on demonstrations of cutting-edge medical technologies developed through federally funded research. Invited guest and Massachusetts State representative, Tommy Vitolo, witnessed the transformative impact of biomedical engineering on healthcare and engaged with AIMBE Fellows during the exhibition and reception. AIMBE strives to enhance collaboration between policymakers and the scientific community to increase federal, state, and local support for medical and biological engineering research.



AIMBE Hosts Congressional Staff Tour of NIH
AIMBE recently hosted its fifth tour of the National Institutes of Health (NIH), providing Congressional staff with a look inside the National Institute of Biomedical Imaging and Bioengineering (NIBIB). Nearly 50 legislative aids to Members of Congress witnessed cutting-edge bioengineering technologies first-hand–the largest Congressional event the NIH has seen to date!
The mission of NIBIB is to improve health by leading the development and accelerating the application of biomedical technologies. The Institute is committed to integrating the physical and engineering sciences with the life sciences to advance basic research and medical care.
By organizing a tour of NIBIB for Congressional staff, AIMBE educates and informs policy makers about the importance of federal funding for biomedical innovation in tackling society’s grand challenges.



AIMBE Hosts Congressional Staff Tour of FDA Labs
AIMBE provided Congressional staff with their first look inside the U.S. Food and Drug Administration (FDA). Nearly 50 legislative aids to Members of Congress toured intramural research labs and met with senior FDA scientists at the Centers for Devices and Radiological Health (CDRH). CDRH Office of Science and Engineering Laboratories (OSEL) Director Edward Margerrison briefed congressional staff about the offices’ mandate to protect and promote the public health by conducting research in order to review cutting-edge medical devices for regulatory approval. CDRH assures that patients and health care providers have timely and continued access to safe, effective, and high-quality medical devices. By organizing a tour of FDA for Congressional staff, AIMBE educates policy makers about the importance of federal funding for medical device research designed to keep Americans safe and healthy. This tour, and AIMBE’s congressional tours of NIH, are part of AIMBE’s expanded advocacy efforts to inform key House and Senate staffers about the role of federal funding for medical device innovation, from the early stages of discovery funded by NIH, to regulatory approval by FDA.


BRINGING YOUR VOICE TO WASHINGTON
AIMBE Highlights Heath AI for Congress
AIMBE hosted a Congressional Lunch Briefing for legislative staffers on AI to Improve Health Care focusing on the risks, opportunities, and challenges of Medical AI. Speakers included AIMBE Fellows, Dr. Regina Barzilay (MIT), and Dr. Parisa Rashidi (University of Florida). As Congress begins to consider further legislation and policy proposals to regulate AI, AIMBE is honored to be at the table providing the medical and biological engineering perspective to lawmakers. Drs. Barzilay and Rashidi were also able to participate in individual meetings with their Senate and Member offices to dive deeper into the issues raised during the briefing.
Following the AIMBE congressional briefing on Health AI, AIMBE submitted written testimony to the Senate Finance Committee in response to their hearing on “Artificial Intelligence and Health Care: Promise and Pitfalls.” Click here to watch a recording of the hearing. Click here to read AIMBE’s testimony.



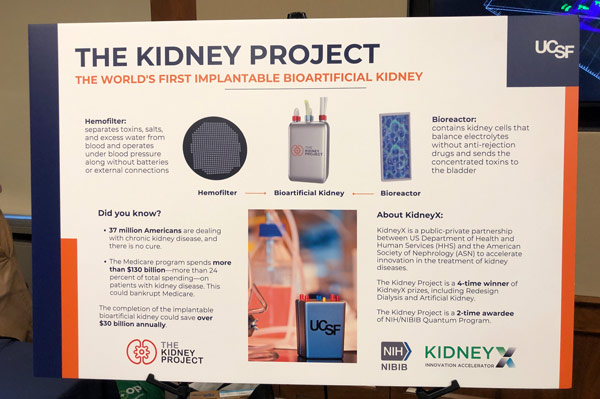
AIMBE Advocates for KidneyX
AIMBE participated in a Congressional Science Exhibition sponsored by the Coalition for Life Sciences in the U.S. House of Representatives. AIMBE highlighted the KidneyX program, a public-private partnership between the Dept. of Health and Human Services (HHS) and the American Society of Nephrology (ASN) designed to spur private sector innovation in kidney disease treatments.
AIMBE Fellow Dr. Shuvo Roy from the University of California, San Francisco demonstrated his KidneyX-funded work on the artificial kidney. This congressional event allowed Members of Congress and their staff to learn about emerging technologies in the life sciences sector supported by federal funding. Dr. Roy met individually with his delegation and co-chairs of the Congressional Kidney Caucus.


Insights from an AIMBE Workshop: Diversifying Paths to Academic Leadership
Abstract
The American Institute for Medical and Biological Engineering (AIMBE) hosted a virtual symposium titled “Diversifying Paths to Academic Leadership.” The symposium sought to educate the community on the opportunities for and impact of leadership by biomedical engineering faculty, to encourage and invite women faculty, especially women of color, to consider and prepare to pursue leadership roles, to educate faculty on the expectations and duties of these roles, and to highlight experiences and paths to leadership of women engineering leaders. Here we review the main outcomes of the symposium to provide perspective on (1) personal visioning and positioning for leadership, (2) negotiating for success in leadership positions, and (3) leadership strategies for success specific to women faculty and where applicable, faculty of color.
AIMBE Focuses on Combating the Opioid Epidemic
AIMBE partners with the Congressional Research and Development Caucus and the Congressional Addiction, Treatment, and Recovery Caucus for a two-part lunch briefing series on combating the opioid epidemic. Biomedical engineers have many tools to help prevent drug abuse by treating pain efficiently and effectively. Dr. Lori Setton, Chair of Biomedical Engineering at Washington University in St. Louis, discusses drug delivery methods that allow for localized pain medication, avoiding oral opioids altogether. Neuromodulation is also an effective strategy to treat pain without oral opioids, which Dr. Walt Baxter and Dr. Tim Denison of Medtronic present to Congress. Dr. Christina Smolke, Professor of Bioengineering at Stanford University, is working to transform the discovery, manufacture, and distribution of opioids through synthetic biology.
AIMBE Advocates for NSF
As an organizational member of the Coalition for National Science Funding (CNSF)–an alliance of over 140 professional organizations, universities and businesses–AIMBE supports the goal of increasing the national investment in the National Science Foundation’s research and education programs in response to the unprecedented scientific, technological and economic opportunities facing the United States.
AIMBE participated in a Congressional Exhibition for Members of Congress and their staff demonstrating the importance of NSF-funded research. AIMBE Fellows Dr. Sarit Bhaduri and Dr. Vijay Goel from the University of Toledo presented their work on healing spine injuries with next-generation bioengineering technology, and met with their delegation while in Washington, DC.



AIMBE Showcases BME Research to Congress
AIMBE participated in a science exhibition sponsored by the Coalition for Life Sciences at the U.S. House of Representatives. The exhibition provided an opportunity for the biomedical research community to engage Members of Congress and their staff about how scientific progress advances public health. AIMBE selected Fellows Dr. Walter Block (University of Wisconsin-Madison) and Dr. Stephanie Fraley (UCSD) to demonstrate their work and participate in congressional visits. AIMBE’s booths featured work on a rapid diagnostic test identifying known and novel bacterial, fungal, and viral pathogens by Dr. Fraley, as well as surgical devices for accelerating magnetic resonance image-guided brain surgery using SBIR funding by Dr. Block.




AIMBE Hosts Congressional Lunch Briefings
AIMBE regularly partners with the Congressional Research and Development Caucus to host an annual series of Congressional Lunch briefings for Members of Congress and their staff highlighting the latest, innovative medical and biological engineering advancing health care. The purpose of the briefing series is to demonstrate breakthroughs only possible with federal funding for R&D and to advocate for increased support.

AIMBE Voice Paper on Making Case for Public Investments in Medical-Technology
A new AIMBE Voice opinion paper recently published by Science Translational Medicine argues Americans have always embraced innovation but today there is a failure in making the case for public investments in medical-technology. AIMBE Fellow David Williams, and Professor of Biomaterials at Wake Force, led a task force in drafting the AIMBE Voice paper. The Task Force included David Williams, Elazer Edelman, Milica Radisci, Cato Laurencin and Darrel Untereker. The paper concludes Americans are less sure the benefits of medical innovation and suggest it is up to the medical-technology sector to educate society in an unbiased rational way about the success and benefits of biotechnology innovation. Only education, communications, and active outreach to and empowering of the community can address sources of tension and correct misunderstanding.
AIMBE Storms the Hill to Advocate for Medical and Biological Engineering
Each year during AIMBE’s Annual Event Fellows across the country travel to Washington, DC to meet with their Representatives and Senators to advocate the positive impact medical and biological engineering has on society. Each Fellow relays their personal experience, whether in the lab, classroom or boardroom of how biomedical engineers are not only creating jobs, but a higher quality of life for all Americans.
Bringing with them medical devices and technologies, Fellows illustrated how federal investment in bioengineering research and development translates ideas from the lab bench to the bedside of patients. Even in these fiscally constrained times, there is a direct need for sustained investment in federal R&D, as slashed funding risks forfeiting America’s leadership in the biotechnology industry to Europe or China. In addition, AIMBE Fellows conveyed the reality that erratic and slashed funding impedes the innovation pipeline, as universities and businesses lose the researchers and the brain power vital to the process of product development. At a time when health care costs continue to soar and manufacturing is shipped overseas, biomedical engineers are the entrepreneurs and innovators who are developing the products that lower health costs and are produced right here in the United States.
By visiting one-on-one, AIMBE Fellows make a powerful statement to our policy makers in Washington how medical and biological engineers are fostering healthy lives, a health economy and a healthy world.


AIMBE Looks Forward to the Next 20 Years
An invited paper published by IEEE focuses on the promises and challenges of the next 20 years in medical and biological engineering. Six overarching challenges were identified and they include: 1) engineering safe and sustainable water and food supply, 2) engineering personalized health care, 3) engineering solutions to injury and chronic diseases, 4) engineering global health through infectious disease prevention and therapy, 5) engineering sustainable bioenergy production, and 6) engineering the 21st century US economy. AIMBE Fellow Robert Linsenmeier, PhD led a task force in drafting the paper. The Task Force included George Pantalos, PhD, Walter Baxter, PhD, Arthur Tipton, PhD, Arthur Coury, PhD, Warren Grundfest, MD, Luis Kun, PhD, Ronald E. Yoder, PhD, William Bentley, PhD, John Watson, PhD, David Jones, Martha Bidez, PhD, Alan Russell, PhD, Thomas Skalak, PhD, Kenneth Lutchen, PhD, and Raphael Lee, MD, ScD.
 AIMBE
AIMBE






