
David Baker, Ph.D.
AIMBE College of Fellows Class of 2020 For outstanding contributions to molecular modeling and protein design platforms that address 21st century challenges in medicine, energy and technology.
They cracked the code for proteins’ amazing structures
Via The Nobel Foundation | October 9, 2024 The Nobel Prize in Chemistry 2024 is about proteins, life’s ingenious chemical tools. David Baker has succeeded with the almost impossible feat of building entirely new kinds of proteins. Demis Hassabis and John Jumper have developed an AI model to solve a 50-year-old problem: predicting proteins’ complex structures. These discoveries hold enormous potential.
The Nobel Prize in Chemistry 2024 is about proteins, life’s ingenious chemical tools. David Baker has succeeded with the almost impossible feat of building entirely new kinds of proteins. Demis Hassabis and John Jumper have developed an AI model to solve a 50-year-old problem: predicting proteins’ complex structures. These discoveries hold enormous potential.
The diversity of life testifies to proteins’ amazing capacity as chemical tools. They control and drive all the chemical reactions that together are the basis of life. Proteins also function as hormones, signal substances, antibodies and the building blocks of different tissues.
“One of the discoveries being recognised this year concerns the construction of spectacular proteins. The other is about fulfilling a 50-year-old dream: predicting protein structures from their amino acid sequences. Both of these discoveries open up vast possibilities,” says Heiner Linke, Chair of the Nobel Committee for Chemistry… Continue reading.
...‘Startling Advance’ in Designer Proteins Opens a World of Possibility for Biotech
Via Singularity Hub | August 16, 2024Proteins are a bit like lights in your house. They have a job to do, and getting them to do it involves switching them on and off with other proteins or molecules.
But it’s much easier to flip the switch on a light. In the body, billions of years of evolution have generated a complex web of molecular signals that act as biological switches for proteins.
This week, a team led by Dr. David Baker at the University of Washington offered a shortcut.
Using AI, they designed proteins that reliably transform themselves in the presence of a molecular switch—dubbed an “effector… Continue reading.
...AI Can Now Design Proteins That Behave Like Biological ‘Transistors’
Via Singularity Hub | August 22, 2023We often think of proteins as immutable 3D sculptures.
That’s not quite right. Many proteins are transformers that twist and change their shapes depending on biological needs. One configuration may propagate damaging signals from a stroke or heart attack. Another may block the resulting molecular cascade and limit harm.
In a way, proteins act like biological transistors—on-off switches at the root of the body’s molecular “computer” determining how it reacts to external and internal forces and feedback. Scientists have long studied these shape-shifting proteins to decipher how our bodies function… Continue reading.
...Protein-Designing AI Opens Door to Medicines Humans Couldn’t Dream Up
Via Singularity Hub | July 26, 2022Designing a protein is a bit like making a cabinet. The first step is building the backbone that holds the protein together. But then comes the hard part: figuring out where to install hinges on the scaffold—that is, finding the best “hotspots”—to put on doors, shelves, and other attachments that ultimately make the cabinet fully functional.
In a way, proteins also have hotspots embedded in their structures. True to their name, “functional sites,” these intriguing nooks and crannies form intricate docks for other proteins or drugs to grab onto. The sites are central to performing most of our basic biological processes. They’re also a massive gold mine for designing new treatments and medical drugs… Continue reading.
...This Algorithm Designs Proteins From Scratch to Accelerate Drug Discovery
Via Singularity Hub | March 24, 2022The proteins that control our lives are like rolling tumbleweeds. Each has a tangled, unique shape, with spiky side-branches dotting its surface. Hidden in the nooks and crannies are the locks to battle our most notorious foes—cancer, diabetes, infections, or even aging—if we can find the right key.
We just got a universal key maker. In a study published today in Nature, a team led by Dr. David Baker from the University of Washington developed an algorithm to design tiny protein keys that unlock those targets from scratch. Far from an ivory tower pursuit, the algorithm tackled one of the most head-scratching drug discovery challenges of our times: can we design drugs based on the structure of a protein’s lock alone… Continue reading.
...Artificial Intelligence Successfully Predicts Protein Interactions
Via Lab Manager | November 17, 2021University of Texas (UT) Southwestern and University of Washington researchers led an international team that used artificial intelligence (AI) and evolutionary analysis to produce 3D models of eukaryotic protein interactions. The study, published in Science, identified more than 100 probable protein complexes for the first time and provided structural models for more than 700 previously uncharacterized ones. Insights into the ways pairs or groups of proteins fit together to carry out cellular processes could lead to a wealth of new drug targets.
“Our results represent a significant advance in the new era in structural biology in which computation plays a fundamental role,” said Qian Cong, PhD, assistant professor in the Eugene McDermott Center for Human Growth and Development with a secondary appointment in Biophysics.
Cong led the study with David Baker, PhD, professor of biochemistry and Cong’s postdoctoral mentor at the University of Washington prior to her recruitment to UT Southwestern… Continue reading.
...Protein biosensors show promise for SARS-CoV-2 testing
Via Science Board | February 1, 2021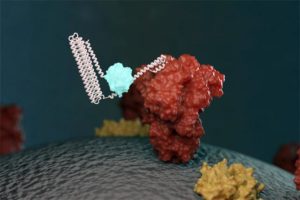
An illustration of a new biosensor binding to a targeted molecule and emitting light. Image courtesy of Ian Haydon/Institute for Protein Design at the University of Washington.
Scientists have developed biosensors to detect SARS-CoV-2 proteins and antibodies in simulated nasal fluids and human sera, according to a study published in Nature on January 27. The approach promises to be less costly and time-consuming than current COVID-19 testing methods.
Biosensors are devices used to detect the presence or concentration of specific biomolecules or biological structures. In this case, the researchers designed protein-based biosensors that recognize specific molecules on the surface of a particular virus and bind to them, then emit light through a biochemical reaction.
The scientists applied this approach to design biosensors of antibodies against SARS-CoV-2 protein epitopes and of the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein.
The result was a biosensor that glows when mixed with COVID-19 antibodies… Continue reading.
...Dr. David Baker Inducted into AIMBE College of Fellows
Via AIMBE | March 30, 2020WASHINGTON, D.C. — The American Institute for Medical and Biological Engineering (AIMBE) has announced the induction of David Baker, Ph.D., Professor, Biochemistry, University of Washington, Investigator, Howard Hughes Medical Institute, to its College of Fellows.
Election to the AIMBE College of Fellows is among the highest professional distinctions accorded to a medical and biological engineer. The College of Fellows is comprised of the top two percent of medical and biological engineers. College membership honors those who have made outstanding contributions to “engineering and medicine research, practice, or education” and to “the pioneering of new and developing fields of technology, making major advancements in traditional fields of medical and biological engineering, or developing/implementing innovative approaches to bioengineering education.”
Dr. Baker was nominated, reviewed, and elected by peers and members of the College of Fellows for “outstanding contributions to molecular modeling and protein design platforms that address 21st century challenges in medicine, energy and technology.“
... AIMBE
AIMBE
