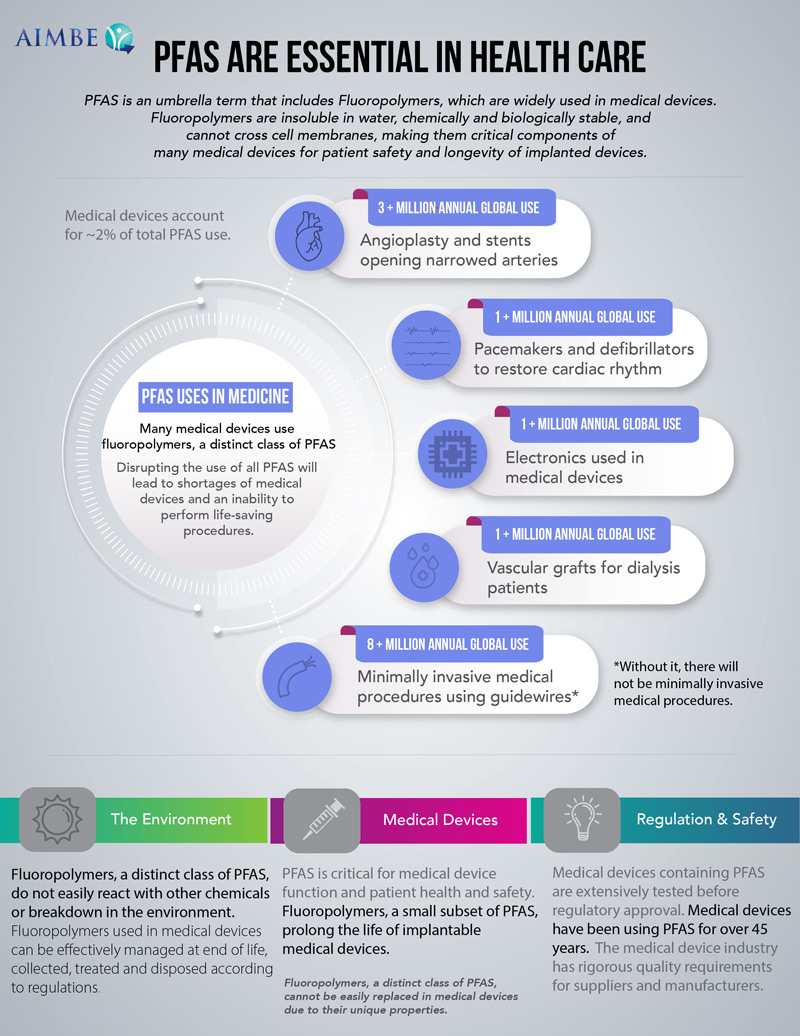
AIMBE’s Industry Council, chaired by Dr. Utkan Demirci, is leading the charge to highlight the importance of Per- and Polyfluorinated Substances (PFAS) to the medical device industry. These so-called “forever chemicals” are rightfully being scrutinized to protect our health and environment; however, a very small number of these chemicals play critical roles in making sure that our medical devices save tens of millions of patients’ lives. AIMBE hosted a roundtable meeting to gather stakeholders to discuss the use of PFAS in medical devices and how they may be impacted by supply and pending regulatory changes. Perspectives including suppliers/processors of PFAS, medical device companies (users of PFAS), federal agencies (e.g., FDA, NIH, NIST, EPA, etc.), academia, public health, policy, and more were represented. The goal of the session was to increase the understanding and awareness of the impacts of PFAS supply and regulatory changes across these different landscapes. A congressional lunch briefing, hosted by AIMBE, was held on the same topic to educate lawmakers and staffers about the importance of fluorinated polymers to live-saving medical devices.
At the state level, AIMBE Fellows, Dr. Nadine Ding (Abbott) and Dr. Bill Wagner (University of Pittsburgh), presented to the Maine State Chamber of Commerce on the importance of PFAS in medical devices in the context of Maine’s PFAS in Products Law which set a ban on all products containing intentionally added PFAS starting in 2030. Dr’s. Ding and Wagner discussed the impact this law would have on medical devices and patients. Following their impactful comments, on April 16, 2024, Maine Governor Janet Mills approved significant revisions to the PFAS in Products Law. The amendments establish exemptions from the law for specific products containing intentionally added PFAS. Importantly, medical devices were included on the list of exemptions.
 AIMBE
AIMBE
 Utkan Demirci, Ph.D.
Utkan Demirci, Ph.D. SuPing Lyu, Ph.D.
SuPing Lyu, Ph.D. Virginia Giddings, Ph.D.
Virginia Giddings, Ph.D. Nadine Ding, Ph.D.
Nadine Ding, Ph.D. M. Allen Northrup, Ph.D.
M. Allen Northrup, Ph.D. Susan Drapeau, Ph.D.
Susan Drapeau, Ph.D. Walt Baxter, Ph.D.
Walt Baxter, Ph.D.
 Robert J. Miller, Ph.D.
Robert J. Miller, Ph.D. Joseph C. Salamone, Ph.D.
Joseph C. Salamone, Ph.D.

