Call Your Lawmaker
Request a Meeting
Visit Your Representative
Write a Letter
Write Your
Lawmaker
Share Grant Disruptions
Share Research Disruptions
Take Action! Resources for Science and Engineering Advocacy
- Tell your Members of Congress how you feel about budget cuts to medical innovation and discovery. Writing a letter to your lawmakers takes 2 minutes.
- Request a meeting with your lawmakers in Washington D.C. Visiting with your Members of Congress is simple. Learn about the process here.
- Invite your lawmakers to tour your lab. Demonstrate the importance of federal funding on your campus, with a tour of your lab for your Members of Congress. Check-out a case study of a lab tour hosted by an AIMBE Fellow.
State and Local Advocacy Resources
Prepare for meetings with your Members of Congress by gathering state-specific data, below, on the federal agencies that support your research.
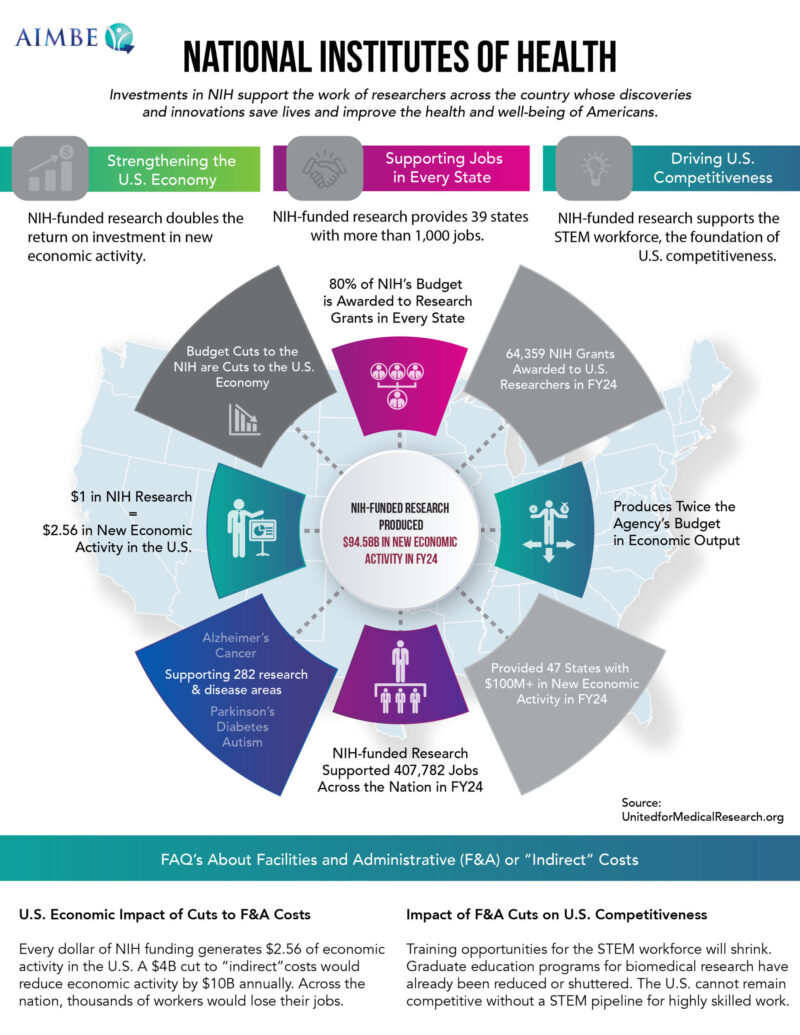
- State Fact Sheets: NIH (United for Medical Research)
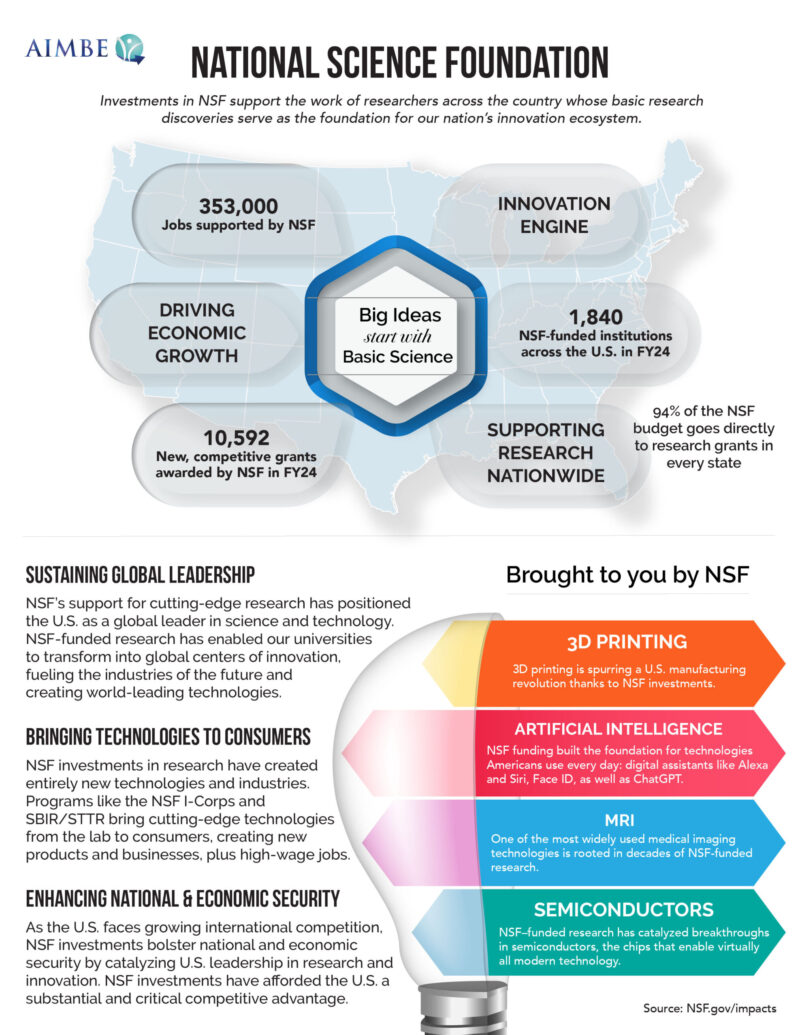
- State Fact Sheets: NSF (National Science Foundation)
- Grant Witness (Previously Grant Watch): A project to track the termination of scientific research grants under the Trump administration in 2025. They are tracking terminations of grants from NIH and NSF
- OpenOMB: Database for Tracking Withholding of Funds to Federal Science Agencies (Protect Democracy Project)
- NIH Grant Terminations by State (Association of American Medical Colleges)
- Federal R&D Investment Visualizer by State for NSF, DOE, NASA, and DOD (American Physical Society)
- FY2026 R&D Appropriations Dashboard (American Association for the Advancement of Science)
- JAG – Financial Accountability in Research Model (FAIR) Indirect Costs Model LinkTree (JAG: Joint Associations Group on F&A Costs)
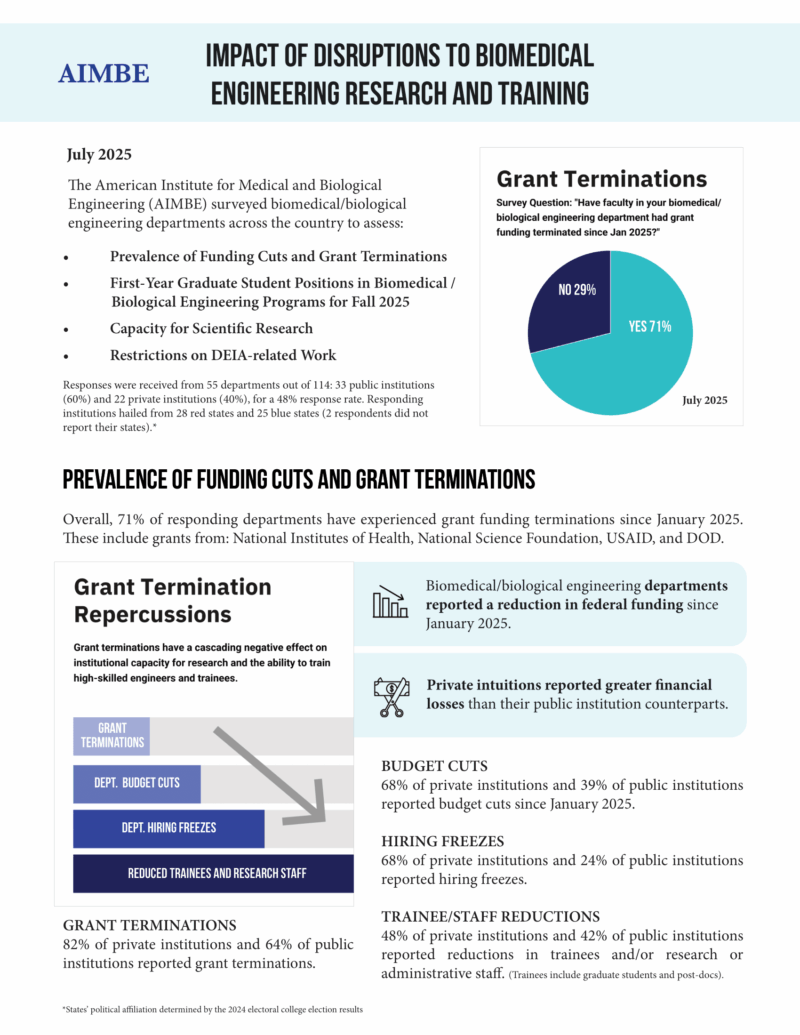
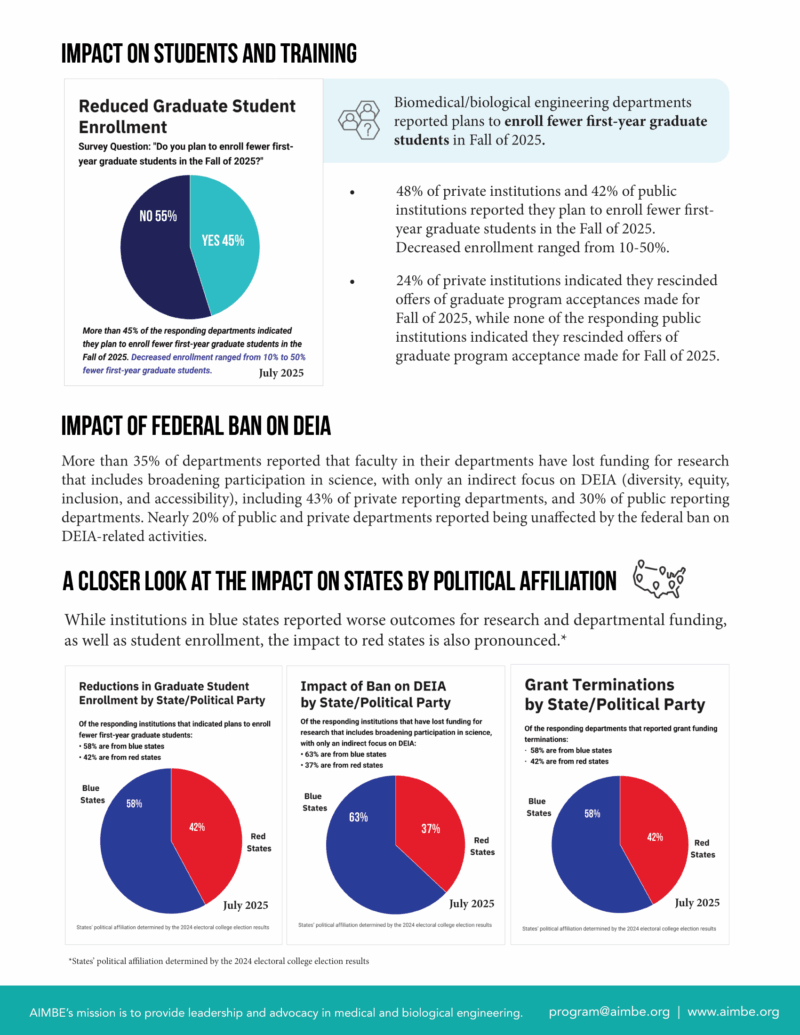
AIMBE Survey Data on Impact of Research Disruptions
AIMBE has released new data on the impact of disruptions to biomedical engineering research and training. AIMBE surveyed over 100 biomedical engineering departments across the country, making us uniquely positioned to gather critical, nationwide insights from academic leaders in the field.
AIMBE Educational One-Pager on New Approach Methodologies (NAMs)
With recent announcements by the FDA and NIH to prioritize human-based research and reducing animal use, AIMBE has developed an educational one-pager on New Approach Methodologies (NAMS) that overview innovations in alternatives to animal testing in biomedical research. This one-pager highlights the definition and types of NAMs, their application areas, and opportunities for their continued development, validation, and commercialization to provide personalized treatment to improve health outcomes.


AIMBE is a Champion for Medicine and Engineering
Among AIMBE’s most important roles is the promotion of public policies that foster continued advancement in medical and biological engineering (MBE). We educate public officials, regulators, the media and general public about the positive impact MBE has on virtually every sector of society. AIMBE advocates for public policy solutions that assistant our community at each stage of the innovation ecosystem–from the research lab to the bedside of a patient.
Bringing Engineers to Washington
- Showcasing Biotech Innovations on Capitol Hill. AIMBE highlights the importance of federal science funding by providing Congressional staff with live bioengineering demos through an interactive exhibition.
- AIMBE Advocates for NSF. AIMBE is a member of the Coalition for National Science Funding–an alliance of over 140 professional organizations and universities–and advocates for increasing federal investments in NSF.
- AIMBE Congressional Lunch Briefings. AIMBE hosts a series of Congressional Lunch Briefings for Members of Congress and their staff highlighting ground-breaking medical and biological engineering research.
- AIMBE Storms the Hill to Advocate for MBE. Each year during AIMBE’s Annual Event, Fellows from across the country travel to Washington, DC to meet with their lawmakers and advocate for medical and biological engineering.
Making the Case for Federal Investments in Engineering and Medicine
- AIMBE Congressional Staff Tours of NIH. AIMBE provides Congressional staff with their first look inside the NIH, witnessing cutting-edge bioengineering technologies first-hand at the National Institute of Biomedical Imaging and Bioengineering (NIBIB)–the largest Congressional event the NIH has seen to date!
- Making the Case for Public Investments in Medical Technology. An AIMBE Voice opinion paper published by Science Translational Medicine argues Americans have always embraced innovation but today there is a failure in making the case for public investments in medical-technology.
- AIMBE Congressional Staff Tours of FDA. This tour, and AIMBE’s congressional tours of NIH, are part of AIMBE’s expanded advocacy efforts to inform key House and Senate staffers about the role of federal funding for medical device innovation, from the early stages of discovery funded by NIH, to regulatory approval by FDA.
Educating the Next Generation on Public Policy
- Educating Graduate Students about Public Policy. AIMBE hosts a 2-day workshop for graduate students to learn from Washington insiders about how public policies impact biomedical engineering innovation.
 AIMBE
AIMBE