AIMBE Fellows' Efforts to Combat COVID-19 Outbreak AIMBE Fellows and the medical and biological engineering community have been deeply involved in responding to the COVID-19 pandemic, and we are honored to share and recognize their efforts.
Broad-spectrum coronavirus drug developed through AI-enabled dynamic modeling
Donald Ingber | Via MedicalXpress | June 12, 2025About 30% of all respiratory tract infections are caused by coronaviruses, leading to widespread illnesses and, in some cases, to epidemic and even pandemic outbreaks, as we experienced with the COVID-19 pandemic. Despite the development of groundbreaking technology that enables the design of prophylactic vaccines, access to those vaccines is not equal across the globe, especially in low-resource countries, and also other hesitations prevent their adoption.
In addition, coronavirus variants are emerging that can have higher infectivity and resistance to existing vaccines and antiviral treatments. Therefore, fast-acting antiviral drugs with broad activity against multiple respiratory coronaviruses and the ability to be rapidly distributed as oral treatments are urgently needed… Continue reading.
New AI tool promises faster vaccine development by predicting T cell epitopes
Regina Barzilay | Via News-Medical.net | January 29, 2025An exciting collaboration between the Ragon Institute and the Jameel Clinic at MIT has achieved a significant milestone in leveraging artificial intelligence (AI) to aid the development of T cell vaccine candidates.
Ragon faculty member Gaurav Gaiha, MD, DPhil, and MIT Professor Regina Barzilay, PhD, AI lead of the Jameel Clinic for AI and Health, have published research in Nature Machine Intelligence introducing MUNIS-a deep learning tool designed to predict CD8+ T cell epitopes with unprecedented accuracy. This advancement has the potential to accelerate vaccine development against various infectious diseases… Continue reading.
Study finds persistent infection could explain long COVID in some people
David Walt | Via EurekAlert | October 9, 2024Brigham researchers found people with wide-ranging long COVID symptoms were twice as likely to have SARS-CoV-2 proteins in their blood, compared to those without long COVID symptoms
A persistent infection could explain why some people experience long COVID symptoms, according to a new study led by researchers at Brigham and Women’s Hospital, a founding member of the Mass General Brigham healthcare system. The team found evidence of persistent infection in 43 percent of participants with cardiopulmonary, musculoskeletal or neurologic symptoms symptoms of long COVID. Results are published in Clinical Microbiology and Infection… Continue reading.
Toll-Like Receptor Nanoparticle Adjuvants Drive Vaccine Response
Eric Appel | Via Genetic Engineering & Biotechnology News | August 21, 2024Toll-like receptor (TLR) and saponin adjuvants have each improved vaccine potency and safety. Now, researchers at Stanford University report that combining them in a nanoparticle format improves not only potency, but also durability, target breadth, and degree of virus neutralization.
A modular approach makes it possible to fine-tune adjuvants by mixing and matching saponin nanoparticles (SNPs) and TLR adjuvants in the same nanostructure to elicit tailored immune responses, according to a recent paper by Eric Appel, PhD, associate professor of materials science and engineering at Stanford University, senior author, and Ben Ou, first author and a doctoral student in the Appel Lab… Continue reading.
More types of lung cells can be infected by SARS-CoV-2 than previously thought, scientists report
Evan Snyder | Via News-Medical.Net | July 23, 2024Scientists at Sanford Burnham Prebys, University of California San Diego and their international collaborators have reported that more types of lung cells can be infected by SARS-CoV-2 than previously thought, including those without known viral receptors. The research team also reported for the first time that the lung is capable of independently mustering an inflammatory antiviral response without help from the immune system when exposed to SARS-CoV-2.
This work is especially timely, as cases of COVID-19 are on the rise in the scientists’ hometown of San Diego during a summertime spike. Looking beyond the region, more than half of the states in the country have reported “very high” or “high” levels of infection, according to the Centers for Disease Control and Prevention… Continue reading.
Deep learning model detects COVID-19 infection using lung imaging
Bisi Bell | Via Health IT Analytics | March 26, 2024A deep neural network-based automated detection tool could assist emergency room clinicians in diagnosing COVID-19 effectively using lung ultrasound images.
Johns Hopkins researchers have developed a deep learning-based model to detect COVID-19 infection using lung ultrasound images, according to a study published recently in Communications Medicine.
The automated detection tool uses deep neural networks (DNNs) to identify COVID-19 features in lung ultrasound B-mode images and may help clinicians diagnose emergency department patients more efficiently.
“We developed this automated detection tool to help doctors in emergency settings with high caseloads of patients who need to be diagnosed quickly and accurately, such as in the earlier stages of the pandemic,” said senior author Muyinatu Bell, PhD… Continue reading.
Improving Nanotherapeutic Vaccine Delivery
Chad Mirkin | Via Northwestern University | October 30, 2023Northwestern Medicine scientists have developed a more effective way of creating nanotherapeutic vaccines and medicines, according to a study published in ACS Nano.
“Over the last decade, spherical nucleic acid, or SNA, technology has emerged as a broad therapeutic platform for a wide variety of diseases, including cancer and other illnesses,” said Chad Mirkin, PhD, professor of Medicine in the Division of Hematology and Oncology, the George B. Rathmann Professor of Chemistry at Northwestern’s Weinberg College of Arts and Sciences, and director of the International Institute for Nanotechnology, who was the lead author of the study.
In the Mirkin laboratory, investigators have harnessed this SNA technology in their work to design precision nanomedicines for use in gene regulation and in cancer immunotherapy with limited unwanted side effects through a systematic development process known as rational vaccinology… Continue reading.
Scientists uncover COVID’s weakness
Jiayu Liao | Via University of California | September 21, 2023In a new paper published in the journal Viruses, the UC Riverside research team describes an important discovery. The protein in COVID that enables the virus to make copies of itself, called N, requires the help of human cells to perform its job.
Genetic instructions in our cells are transcribed from DNA to messenger RNA, and then translated into proteins that enable functions such as growth and communication with other cells. Following this translation event, proteins often need additional modifications by enzymes. These so-called post-translation modifications ensure that proteins are uniquely suited to perform their intended tasks… Continue reading.
A low-cost, eco-friendly COVID test
Cesar de la Fuente-Nunez | Via University of Pennsylvania | September 1, 2023César de la Fuente and a team of Penn engineers work on creative ways to create faster and cheaper testing for COVID-19. Their latest innovation incorporates speed and cost-effectiveness with eco-friendly materials.
When it comes to COVID-19 testing, polymerase chain reaction (PCR) tests, are the “gold standard” for diagnostic testing. However, these tests are hampered by waste. They require significant time (results can take up to a day or more) as well as specialized equipment and labor, all of which increase costs. The sophistication of PCR tests makes them harder to tweak, and therefore slower to respond to new variants. They also carry environmental impacts.
In order to balance the need for fast, affordable and accurate testing while addressing these environmental concerns, César de la Fuente, Presidential Assistant Professor in bioengineering and chemical and biomolecular Engineering in the School of Engineering and Applied Science, with additional primary appointments in Psychiatry and Microbiology within the Perelman School of Medicine, has turned his attention to the urgent need for “green” testing materials… Continue reading.
100-Year-Old Treatment Inhibits COVID-19 Infection
Jonathan Dordick | Via Rensselaer Polytechnic Institute | July 24, 2023A team of researchers led by Rensselaer Polytechnic Institute’s Jonathan S. Dordick, Ph.D., Institute Professor of Chemical and Biological Engineering, has illuminated a new possibility for the treatment and prevention of COVID-19 in research published in Communications Biology.
The team found that suramin, a 100-year-old drug still used for human sleeping sickness that has many other potential applications, inhibits the infection of SARS-CoV-2.
“Suramin binds to the ACE2 and cell surface heparan sulfate binding sites on the receptor binding domain (RBD) of the viral spike (S) protein in vitro,” said Dordick… Continue reading.
Artificial Intelligence is Leveling Up the Fight Against Infectious Diseases
Cesar de la Fuente-Nunez | Via University of Pennsylvania | July 20, 2023Artificial intelligence is a new addition to the infectious disease researcher’s toolbox. Yet in merely half a decade, AI has accelerated progress on some of the most urgent issues in medical science and public health. Researchers in this field blend knowledge of life sciences with skill in computation, chemistry and design, satisfying decades-long appeals for interdisciplinary tactics to treat these disorders and stop their spread.
Diseases are “infectious” when they are caused by organisms, including parasites, viruses, bacteria and fungi. People and animals can contract infectious diseases from their environments or food, or through interactions with one another. Some, but not all, are contagious… Continue reading.
A unique study on COVID shows how machine learning can help personalize medicine
Jiayu Liao | Via Pharma Voice | June 26, 2023Based on real-world data from patients in China, researchers were able to pinpoint factors that led to recurring infections — and which drug combos helped.
In the earliest days of the COVID-19 pandemic, doctors in China tried a barrage of drugs to quell the raging virus. In one Shenzhen hospital, treatments included combinations of up to eight antiviral, anti-inflammatory or immune-modulating drugs. But with no way of knowing how well different combinations would work, it was a trial-and-error approach.
Now, years later, a team of scientists at the University of California (UC) Riverside have followed up on these early medical practices and used machine learning to analyze how the various drug combinations performed — while also predicting which ones could keep further COVID-19 infections at bay… Continue reading.
Study shows metformin lowers the risk of getting long COVID
David Odde | Via University of Minnesota | June 9, 2023In a study published in The Lancet Infectious Diseases, University of Minnesota researchers found that metformin, a drug commonly used to treat diabetes, prevents the development of long COVID. The study investigated if early outpatient COVID-19 treatment with metformin, ivermectin, or fluvoxamine could prevent long COVID. A simulator developed by David Odde and team predicted metformin’s ability to stop the SARS-CoV-2 virus… Continue reading.
Microneedle Patch Printer Enables On-Demand Vaccine Manufacturing
Ana Jaklenec | Via Genetic Engineering & Biotechnology News | April 24, 2023The portable instrument could increase global access to vaccines by simplifying their storage, distribution, and administration.
Researchers from the lab of Robert Langer, ScD, at the Koch Institute for Integrative Cancer Research at the Massachusetts Institute of Technology (MIT), say they have developed a printer for microneedle patches smaller than postage stamps that penetrate the skin to deliver vaccines, including the COVID-19 mRNA vaccine.
The research article, “A microneedle vaccine printer for thermostable COVID-19 mRNA vaccines,” was published in Nature Biotechnology… Continue reading.
How entrepreneurship and industry saved COVID fighting nanotechnology?
Tom Webster | Via Open Access Government | April 14, 2023Here Thomas J. Webster, Ph.D. explores how Nanotechnology was crucial in the battle against COVID-19 and how entrepreneurship helped it thrive
COVID changed the world forever. Although viruses are nothing new to mankind, COVID highlighted significant deficiencies in our current global healthcare system
Our healthcare system was ill-prepared for a viral pandemic, as well as many other health crises. Our only recourse when COVID emerged was to shut everything down and tell people not to interact for fear of spreading the virus. Stay home. Cancel trips. Do everything virtually. There are few examples of larger healthcare failures than COVID, when the only way to stop a virus from spreading was to tell people to stop seeing each other… Continue reading.
Research sheds light on protections against COVID-19 variant infections
James Baker | Via Newswise | March 16, 2023Research is shedding light on why ‘breakthrough’ Omicron infections occur in vaccinated individuals and suggests those who are both vaccinated and experienced previous infection have better protection against getting sick again.
The research shows that having both infection and vaccination with the “wild-type” virus, or the original COVID-19 variant, provides individuals with the strongest protection against all variants; those who were unvaccinated or who had not previously had the virus were more likely to have undetectable neutralization against all variants of COVID-19. Immunity provided by vaccines appears to wane over time. The research supports Omicron-specific vaccine boosters to better protect those who have not previously been infected.
“This study shows that immunity from infection, sometimes called ‘natural immunity,’ plays an important role in protection against subsequent COVID-19 infection,” James Baker, Director of the Mary H. Weiser Food Allergy Center, said… Continue reading.
NIH launches at-home COVID-19 test and virtual treatment program
Bruce Tromberg | Via Innovate Healthcare - HealthExec | January 6, 2023A new at-home testing and treatment program for COVID-19 is on its way to selected communities.
The program, called the Home Test to Treat program, is being launched by the National Institutes of Health, in collaboration with the Administration for Strategic Preparedness and Response (ASPR) at the U.S. Department of Health and Human Services (HHS). It will provide free COVID-19 health services, including at-home rapid tests, telehealth sessions and at-home treatments. The program was first introduced in September 2022 by the White House and aims to make antiviral treatment available for eligible individuals who receive a positive test result to help prevent severe illness, hospitalization or death.
The program launch comes as the United States is grappling with a “tripledemic,” with rising cases of COVID-19, influenza and respiratory syncytial virus (RSV). There are currently more than 470,000 new weekly cases of COVID-19 in the United States, according to the latest data from the Centers for Disease Control and Prevention… Continue reading.
Researchers Hunt Biomarkers – Potential Keys to Long COVID
David Walt | Via WebMD | January 6, 2023Even if the causes of long COVID remain confusing, researchers are zeroing in on biomarkers – compounds that can be detected and measured – that can help them better diagnose and treat the condition. The eventual goal: a simple test to help determine who has long COVID and whether treatments are helping.
“The hope is that the specific markers that are discovered will inform how individual clusters (of disease) should be treated and managed to either reduce or eliminate symptoms,” says David Walt, PhD, co-director of the Mass General Brigham Center for COVID Innovation in Boston… Continue reading.
Investigators find lab-grown retinal eye cells make key connections, open door for clinical trials to treat blindness
David Gamm | Via Modern Retina | January 6, 2023Retinal cells grown from stem cells can reach out and connect with neighbors, according to a new study, completing a “handshake” that may show the cells are ready for trials in humans with degenerative eye disorders.
According to a news release, more than a decade ago, researchers from the University of Wisconsin–Madison developed a way to grow organized clusters of cells, called organoids, that resemble the retina, the light-sensitive tissue at the back of the eye. They coaxed human skin cells reprogrammed to act as stem cells to develop into layers of several types of retinal cells that sense light and ultimately transmit what we see to the brain… Continue reading.
Young Adults Develop Rare Complication After COVID Vaccine: Study
David Walt | Via Mirage News | January 4, 2023Myocarditis, a condition in which the heart muscle becomes inflamed, is a rare complication that can occur after mRNA COVID vaccination. It’s estimated that roughly 18 cases occur in every 1 million vaccine doses administered, making it so rare that it is challenging to find cases to investigate. In a new study by researchers from Mass General Brigham‘s founding members, Brigham and Women’s Hospital and Massachusetts General Hospital, a team extensively investigated the immune response of 16 adolescents and young adults who developed myocarditis after receipt of the COVID mRNA vaccine. The researchers found no differences in antibody production, auto-antibodies, T cell profiles, or prior viral exposures, but found elevated levels of spike protein along with increased cytokines (consistent with innate inflammation) and increased troponin (indicating cardiac injury). Their results are published in Circulation… Continue reading.
Are Covid-19 “comas” signs of a protective hibernation state?
Emery Brown | Via MIT | November 18, 2022Scientists hypothesize that, as in a hibernating turtle, the brain under sedation and deprived of oxygen may assume a protective state.
Many Covid-19 patients who have been treated for weeks or months with mechanical ventilation have been slow to regain consciousness even after being taken off sedation. A new article in the Proceedings of the National Academy of Sciences offers the hypothesis that this peculiar response could be the effect of a hibernation-like state invoked by the brain to protect cells from injury when oxygen is scarce.
A very similar kind of state, characterized by the same signature change of brain rhythms, is not only observed in cardiac arrest patients treated by chilling their body temperature, a method called “hypothermia,” but also by the painted turtle, which has evolved a form of self-sedation to contend with long periods of oxygen deprivation, or “anoxia,” when it overwinters underwater… Continue reading.
Finding the “Sweet Spot” for Indoor Humidity May Help to Reduce COVID-19 Transmission
Lydia Bourouiba | Via Infectious Disease Special Edition | November 18, 2022As friends and families are beginning to plan holiday gatherings, a new study found that raising the humidity level could be another mitigation method to reduce COVID-19. That sweet spot looks to be between 40% and 60% humidity.
Researchers from Massachusetts Institute of Technology (MIT) combined population-based COVID-19 data with meteorologic measurements from 121 countries collected between January and August 2020 (J R Soc Interface 2022;19[196]:20210865). Countries included had reported at least 50 COVID-19–related deaths, indicating at least one outbreak had occurred. The researchers processed the epidemiological data while accounting for bias, and developed a computational workflow to estimate indoor conditions based on outdoor weather data and standard indoor comfort conditions… Continue reading.
What causes severe COVID symptoms? Research examines role of immune systems
Melody Swartz | Via UChicago News | October 20, 2022UChicago study examines how autoantibodies could cause complications in some patients
Since the earliest months of the COVID-19 pandemic, physicians and scientists worldwide have been working to understand how exactly the virus makes us sick. That task, already complicated by COVID’s rapid spread, is made more challenging by some of its unusual, seemingly inexplicable symptoms, such as blood pressure dysregulation and blood clots.
Now, research from the University of Chicago’s Pritzker School of Molecular Engineering (PME) shows that the immune system may unintentionally contribute to the disease’s strangest symptoms… Continue reading.
Researchers Use Genetic Sequencing and Wastewater Analysis to Detect SARS-CoV-2 Variants and Monkeypox within Communities
Rob Knight | Via Dark Daily | September 12, 2022Researchers surprised that process designed to detect SARS-CoV-2 also identifies monkeypox in wastewater
Early information about an outbreak in a geographical region can inform local clinical laboratories as to which infectious agents and variants they are likely to see when testing patients who have symptoms. To that end, wastewater testing has become a rich source of early clues as to where COVID-19 outbreaks are spreading and how new variants of the coronavirus are emerging.
Now, scientists in San Diego County are adding monkeypox to its wastewater surveillance, according to an August University of California San Diego (UCSD) Health press release. The team at UCSD uses the same process for detecting SARS-CoV-2… Continue reading.
Researchers in Boston Find COVID-19 Spike Protein Lingers in Long COVID-19 Patients
David Walt | Via Dark Daily | August 31, 2022Viral reservoir could be behind persistence, says study, which also suggests a blood biomarker could be found for clinical laboratory testing
Microbiologists and virologists working closely with physicians treating long COVID-19 patients will gain new insights in a study that found coronavirus spike protein in COVID-19 patients’ blood up to 12 months after diagnosis. The researchers believe their findings could be used to develop a clinical laboratory biomarker for long COVID-19.
Researchers at Brigham and Women’s Hospital and Massachusetts General Hospital said medical experts are not sure why some people have unwelcome symptoms weeks and months after a positive COVID-19 diagnosis, while others clear the infection without lingering effects… Continue reading.
Microparticles could be used to deliver “self-boosting” vaccines
Ana Jaklenec | Via MIT | July 13, 2022Most vaccines, from measles to Covid-19, require a series of multiple shots before the recipient is considered fully vaccinated. To make that easier to achieve, MIT researchers have developed microparticles that can be tuned to deliver their payload at different time points, which could be used to create “self-boosting” vaccines.
In a new study, the researchers describe how these particles degrade over time, and how they can be tuned to release their contents at different time points. The study also offers insights into how the contents can be protected from losing their stability as they wait to be released… Continue reading.
Microparticles could be used to deliver “self-boosting” vaccines
Robert Langer | Via MIT | July 13, 2022Most vaccines, from measles to Covid-19, require a series of multiple shots before the recipient is considered fully vaccinated. To make that easier to achieve, MIT researchers have developed microparticles that can be tuned to deliver their payload at different time points, which could be used to create “self-boosting” vaccines.
In a new study, the researchers describe how these particles degrade over time, and how they can be tuned to release their contents at different time points. The study also offers insights into how the contents can be protected from losing their stability as they wait to be released… Continue reading.
When it comes to darker skin, pulse oximeters fall short
Kimani Toussaint | Via NPR | July 11, 2022Over the past two years, the pulse oximeter has become a crucial tool for tracking the health of COVID-19 patients.
The small device clips onto a finger and measures the amount of oxygen in a patient’s blood. But a growing body of evidence shows the device can be inaccurate when measuring oxygen levels in people with dark skin tones.
A study published on Monday only adds to this concern.
Researchers analyzing pre-pandemic health data also find those measurements resulted in patients of color receiving less supplemental oxygen than white patients did… Continue reading.
Inhalable COVID-19 Vaccine Shows Promise in Rodent Model
Ke Cheng | Via NC State University | July 5, 2022Researchers have created an inhalable COVID-19 vaccine that is shelf stable at room temperature for up to three months, targets the lungs specifically and effectively, and allows for self-administration via an inhaler. The researchers also found that the delivery mechanism for this vaccine – a lung-derived exosome called LSC-Exo – is more effective at evading the lung’s mucosal lining than the lipid-based nanoparticles currently in use, and can be used effectively with protein-based vaccines.
Ke Cheng, the Randall B. Terry Jr. Distinguished Professor in Regenerative Medicine at NC State and a professor in the NC State/UNC-Chapel Hill Joint Department of Biomedical Engineering, along with colleagues from UNC-Chapel Hill and Duke University, led the development of the vaccine prototype from proof-of-concept to animal studies… Continue reading.
Understanding Poor Vaccine Responses in Individuals With Weakened Immune Systems
Rong Fan | Via Yale School of Medicine | June 16, 2022When the COVID-19 vaccine first became available, people eagerly signed up for coveted slots to boost their antibodies against the virus that shut down much of the world. But not everyone who rolled up their sleeve received equal protection from illness.
A significant proportion of the population has weakened immune systems, including cancer patients, those with autoimmune disease, and organ transplant recipients. The immune systems of these individuals are unable to ward off disease as effectively, and their responses to vaccines are not as robust. Now, Yale researchers have received a $12 million award from the NIH as part of the Human Immune Project Consortium (HIPC) to study vaccine responses in vulnerable groups, including patients with multiple sclerosis (MS) undergoing B cell depletion therapy, older adults including particularly vulnerable older residents of long-term care facilities, and individuals with sickle cell disease who have substantial and potentially disabling morbidity and early mortality for whom fundamental challenges to improve clinical outcomes remain… Continue reading.
No more flu for you? Discovery blocks influenza virus’ replication in cells
Jiayu Liao | Via University of California, Riverside | May 31, 2022SUMOylation inhibitor could lead to highly effective ways to treat the flu and other respiratory viruses
It happens every year, especially in winter. A virus saunters into your wide-open respiratory tract, worms its way into lung cells, and, next thing you know, you’re lying in bed with a fever, aches, and chills—classic symptoms of influenza, or flu.
Research led by UC Riverside bioengineers may help stop that cycle. The team has just found a way to block one strain of the influenza virus from accessing a human protein it needs to replicate in cells. The discovery could lead to highly effective ways to treat the flu and could also apply to other respiratory viruses, such as SARS-CoV-2, which causes Covid-19… Continue reading.
Novel Immunotherapy Developed by City of Hope Could Provide New Treatment Model for SARS-CoV-2 Patients
Michael Caligiuri | Via BioSpace | May 20, 2022City of Hope researchers have engineered an immunotherapy using natural killer cells with a specific molecule that can target the SARS-CoV-2 virus’ spike protein, providing a novel therapeutic pathway for the treatment of COVID-19 and other infections that include the spike protein, according to a study published in Nature Communications. The research adds to City of Hope’s leadership in using CAR T cell therapy, natural killer cells and other immunotherapies to help find better treatments against cancer and other diseases.
“The importance of this off-the-shelf therapy is that one does not need to use one’s own cells — the cells can be frozen and ready to go, locally or shipped anywhere around the world,” said Michael Caligiuri, M.D., president of City of Hope National Medical Center, the Deana and Steve Campbell Physician-in-Chief Distinguished Chair and one of the study’s authors… Continue reading.
Study estimates effectiveness of 2-dose and 3-dose mRNA vaccination against Omicron
Delphine Dean | Via News-Medical.Net | May 12, 2022In a recent study posted to the medRxiv* preprint server, researchers estimated the efficacy of two-dose and three-dose regimens of two messenger ribonucleic acid (mRNA) vaccines: Moderna’s mRNA-1273 and Pfizer-BioNTech’s BNT162b2 against coronavirus disease 2019 (COVID-19) caused due to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant.
Omicron (B.1.1529) has demonstrated higher infectivity compared to other SARS-CoV-2 variants. In addition, studies have reported lower Omicron neutralization by the existing COVID-19 vaccines. Despite this, it is not clear just how much protection the COVID-19 vaccine confers against Omicron infections… Continue reading.
Rensselaer Professor Jonathan Dordick and Collaborators Work To Develop Nasal Spray To Combat COVID-19 and Other Respiratory Infections
Jonathan Dordick | Via Rensselaer Polytechnic Institute | April 12, 2022Rensselaer Professor Jonathan Dordick and collaborators from Rensselaer Polytechnic Institute and Albany Medical College have been awarded $500,000 from the New York State Biodefense Commercialization Fund to engage in research for the development of a Pentosan Polysulfate (PPS)-based nasal spray to block COVID-19.
“Despite advances in both therapeutics and vaccines for COVID-19, there remains a critical need to develop a simple, easy to use, and highly effective prophylactic to prevent transmission and serious illness as a result of infection with SARS-CoV-2,” said Dr. Dordick, Institute Professor of Chemical/Biological Engineering at Rensselaer, and co-director of the Heparin Applied Research Center (HARC)… Continue reading.
This Algorithm Designs Proteins From Scratch to Accelerate Drug Discovery
David Baker | Via Singularity Hub | March 24, 2022The proteins that control our lives are like rolling tumbleweeds. Each has a tangled, unique shape, with spiky side-branches dotting its surface. Hidden in the nooks and crannies are the locks to battle our most notorious foes—cancer, diabetes, infections, or even aging—if we can find the right key.
We just got a universal key maker. In a study published today in Nature, a team led by Dr. David Baker from the University of Washington developed an algorithm to design tiny protein keys that unlock those targets from scratch. Far from an ivory tower pursuit, the algorithm tackled one of the most head-scratching drug discovery challenges of our times: can we design drugs based on the structure of a protein’s lock alone… Continue reading.
Nanoparticle-Based COVID-19 Vaccine Could Target Future Infectious Diseases
Chad Mirkin | Via Northwestern University | March 23, 2022Just one dose of a new nanoparticle-based COVID-19 vaccine was enough to produce an immune response in animals on track with vaccines currently in clinical use. And with minor changes, Northwestern University investigators hope the same vaccine platform could target other infectious diseases.
In a new study, published in PNAS, 100 percent of mice who received the protein-based immunization survived when challenged with lethal doses of the SARS-CoV-2 virus, which causes COVID-19. None of the mice experienced lung damage due to SARS-CoV-2 exposure, and all mice who did not receive this nanoparticle vaccine died in a 14-day trial… Continue reading.
Researchers identify a promising drug for treating serious COVID-19 complication in children
David Walt | Via EurekAlert | February 23, 2022Scientists at Massachusetts General Hospital (MGH) and Brigham and Women’s Hospital (BWH) have identified a promising drug candidate for the treatment of multi-inflammatory syndrome in children (MIS-C), they report in Clinical Care Explorations. MIS-C is a rare but severe and potentially life-threatening condition that usually develops in children weeks to months after they have experienced a mild or even asymptomatic case of COVID-19.
MIS-C occurs mainly in children and leads to high fevers and a hyperinflammatory response that can affect multiple organs, including the heart, brain and gastrointestinal organs. Symptoms include stomach pain, diarrhea, vomiting, dizziness and rash. Fifty-five of the 6,431 children diagnosed with MIS-C have died since May 2020, according to the Centers for Disease Control and Prevention… Continue reading.
Nasal Approach to COVID Vaccination Gains Traction
Mark Saltzman | Via Yale University | February 14, 2022As the coronavirus that causes COVID-19 continues to mutate, it presents new roadblocks to efforts to contain its spread. A Yale research team led by Akiko Iwasaki, PhD, Waldemar von Zedtwitz Professor of Immunobiology and professor of molecular, cellular, and developmental biology and of epidemiology (microbial diseases), has found success in a new approach to vaccination—systemic vaccines that train the entire body’s immune response followed by boosters administered directly to the nasal cavity, to deliver special protection in the part of the body most affected by SARS-CoV-2 infection.
In a research paper posted on the preprint site bioRxiv, Iwasaki and co-first authors Tianyang Mao, BS, and Benjamin Israelow, MD, PhD, note that the mRNA-based vaccines that have been such a powerful tool against COVID have shown diminished effectiveness over time. They especially appear to lack strength in the nasal cavity mucosa and respiratory tract—the region of the body where the virus is most likely to cause illness and from which it is most likely to be transmitted to other people… Continue reading.
How Omicron escapes from antibodies
Ram Sasisekharan | Via MIT | February 1, 2022A new study from MIT suggests that the dozens of mutations in the spike protein of the Omicron variant help it to evade all four of the classes of antibodies that can target the SARS-CoV-2 virus that causes Covid-19.
This includes antibodies generated by vaccinated or previously infected people, as well as most of the monoclonal antibody treatments that have been developed, says Ram Sasisekharan, the Alfred H. Caspary Professor of Biological Engineering and Health Sciences and Technology (HST) at MIT.
Using a computational approach that allowed them to determine how mutated amino acids of the viral spike protein influence nearby amino acids, the researchers were able to get a multidimensional view of how the virus evades antibodies. According to Sasisekharan, the traditional approach of only examining changes in the virus’ genetic sequence reduces the complexity of the spike protein’s three-dimensional surface and doesn’t describe the multidimensional complexity of the protein surfaces that antibodies are attempting to bind to… Continue reading.
Mathematical model may help improve treatments and clinical trials of patients with COVID-19 and other illnesses
Rakesh Jain | Via Massachusetts General Hospital | January 14, 2022A mathematical model revealed that the optimal time to initiate immune-modulating therapy in COVID-19 differed according to patients’ medical history and risk factors. Different patients also required different types of immunomodulation for optimal therapy.
Certain biological markers that differed based on patient characteristics determined optimal treatment initiation time, and these markers pointed to particular biologic programs or mechanisms that affected a patient’s outcome.
Use of the model may help physicians tailor treatments to different patients and also indicate which patients are most likely to respond to certain drugs tested in clinical trials… Continue reading.
Simultaneous Detection of SARS-CoV-2 and Influenza Viruses at the Point-of-Care
Hugh Fan | Via University of Florida | December 17, 2021With co-circulation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza viruses during the ongoing pandemic and future flu seasons, it is desirable to have an ability to detect these two viruses simultaneously at the point-of-care (POC) for appropriate clinical care and quarantine procedures. To address the need, Hugh Fan and his colleagues have developed… Continue reading.
COVID-19 can infect the inner ear – Scope Blog
Lee Gehrke | Via Stanford University | December 1, 2021Early into the pandemic, Konstantina Stankovic, MD, PhD, an inner ear researcher, and otolaryngologist, was surprised when she began seeing patients exposed to COVID-19 in her Massachusetts clinic complaining of hearing loss, ringing in the ears known as tinnitus, and dizziness, which often starts in the inner ear.
Sure, everyone had heard about other odd sensory side effects, such as loss of taste and smell, because of the disease, but hearing loss? That wasn’t even on most people’s radar.
Now, after months of research which culminated in the recent publication of a study in Communications Medicine which links the coronavirus with hearing and balance disorders, Stankovic believes these symptoms should be on everybody’s radar… Continue reading.
New research NETs a fresh angle for treating severe inflammation
Jeffrey Karp | Via Boston Children’s Hospital | October 27, 2021 As we’ve seen during the COVID-19 pandemic, serious infections sometimes trigger an excessive inflammatory reaction that does as much harm — or more — than the infection itself. New research at Boston Children’s Hospital and Brigham and Women’s Hospital suggests a potential way to block this hyperinflammation response by repurposing or modifying an existing drug.
As we’ve seen during the COVID-19 pandemic, serious infections sometimes trigger an excessive inflammatory reaction that does as much harm — or more — than the infection itself. New research at Boston Children’s Hospital and Brigham and Women’s Hospital suggests a potential way to block this hyperinflammation response by repurposing or modifying an existing drug.
The findings could potentially lead to a new treatment not just for COVID-19, but also for other life-threatening inflammatory conditions like sepsis and acute respiratory distress syndrome (ARDS) that currently have no specific treatment… Continue reading.
These fridge-free, no-needle vaccines could be ready for the next pandemic
Nicole Steinmetz | Via fastcompany | September 14, 2021The COVID-19 vaccine rollout has been mired in logistical challenges. Millions of doses have gone to waste, some spoiling after being left unrefrigerated for too long, some expiring before they could physically get to those in need. Most of the vaccines need to be kept at incredibly low temperatures, requiring a cold chain for distribution, a tricky feat when the vaccine needs to access remote areas or regions without electricity. Even with the vaccines in place, you still need people to put those shots in arms, and to come back and do it all again for the second dose.
Nicole Steinmetz, a professor of nanoengineering and the director of the Center for Nano ImmunoEngineering at the University of California, San Diego (UCSD), imagines another way: “thermally stable” vaccines that don’t need to be transported in freezers, and which could come in a microneedle patch—so that “you could ship it to people’s homes, and they can self-administer just like a bandaid,” she says—or in one-dose implants, eliminating the need to set up a second appointment… Continue reading.
Study sounds note of caution on effectiveness of Covid vaccines for patients with lymphoid malignancies
David Walt | Via Mirage News | August 24, 2021Patients with lymphoma or other lymphoid cancers should continue to take steps to protect themselves from COVID-19 even if they have been vaccinated against the disease, a new study led by investigators at Dana-Farber Cancer Institute reports. The study, published online by the journal Blood Advances, found that patients who had received anti-CD20 antibody therapy within the previous 12 months did not develop protective antibodies for COVID-19 after being vaccinated.
“Our findings suggest that patients with lymphoid cancers who have been vaccinated for COVID-19 should not assume they have immunity against the disease… Continue reading.
Detecting COVID-19 by Analyzing Lung Images Using Artificial Intelligence Models
Ali Khademhosseini | Via Health IT Analytics | August 16, 2021Using artificial intelligence technology, Terasaki Institute for Biomedical Innovation (TIBI) researchers developed and validated an image-based detection model for COVID-19. The model analyzes lung images and can detect COVID-19 infection.
Medical imaging has become an important tool in the diagnosis and prognostic assessments of diseases. In recent years, artificial intelligence models have been implemented with imaging technology to improve diagnostic capabilities. In comporting AI into imaging technology, models can reveal disease characteristics that are not visible to the naked eye… Continue reading.
Open-access database could speed up repurposing of old drugs as new treatments
Vassily Hatzimanikatis | Via News-Medical.Net | August 3, 2021Researchers have created a new open-access database of information on drug candidates and how they are metabolized by the body, which could help speed up the repurposing of old drugs as new treatments.
There is an urgent need for more effective treatments for many conditions, including COVID-19, cancer and malaria. But the process of developing new drugs is costly, can take decades, and often leads to failed treatments. The database, called NICEdrug.ch and described today in eLife, may help expedite the process by helping scientists find promising, existing drugs that might be repurposed for these diseases… Continue reading.
New face mask prototype can detect Covid-19 infection
James Collins | Via MIT | June 28, 2021Engineers at MIT and Harvard University have designed a novel face mask that can diagnose the wearer with Covid-19 within about 90 minutes. The masks are embedded with tiny, disposable sensors that can be fitted into other face masks and could also be adapted to detect other viruses.
The sensors are based on freeze-dried cellular machinery that the research team has previously developed for use in paper diagnostics for viruses such as Ebola and Zika. In a new study, the researchers showed that the sensors could be incorporated into not only face masks but also clothing such as lab coats, potentially offering a new way to monitor health care workers’ exposure to a variety of pathogens or other threats… Continue reading.
Anticancer drug effective against severe COVID-19
Yihai Cao | Via Sciencenews | May 7, 2021A small trial with participants from Italy and China shows that the anticancer drug bevacizumab is extremely effective in reducing severe COVID-19 symptoms.
The whole world is looking for new ways to combat COVID-19.
This mainly relates to manufacturing vaccines, but pharmaceutical companies have also developed many antibody treatments to the clinical trial stage and launched them on the market.
The anticancer drug bevacizumab may be able to lend the world an unexpected helping hand. A new study shows that bevacizumab can significantly reduce severe respiratory symptoms and fever from COVID-19. It can also reduce the risk of dying from COVID-19 and alleviate a severe COVID-19 trajectory… Continue reading.
Sweat sensor could alert doctors, patients to looming COVID cytokine storm
Shalini Prasad | Via ACS | April 16, 2021Early in the COVID-19 pandemic, doctors recognized that patients who developed a “cytokine storm” — a surge of pro-inflammatory immune proteins — were often the sickest and at highest risk of dying. But a cytokine storm can also occur in other illnesses, such as influenza. Today, scientists report preliminary results on a sweat sensor that acts as an early warning system for an impending cytokine storm, which could help doctors more effectively treat patients.
The researchers will present their results today at the spring meeting of the American Chemical Society (ACS). ACS Spring 2021 is being held online April 5-30. Live sessions will be hosted April 5-16, and on-demand and networking content will continue through April 30. The meeting features nearly 9,000 presentations on a wide range of science topics… Continue reading.
Vaccination by inhalation
Darrell Irvine | Via MIT | March 19, 2021Delivering vaccines directly to the lungs can boost immune responses to respiratory infections or lung cancer, study finds.
Many viruses infect their hosts through mucosal surfaces such as the lining of the respiratory tract. MIT researchers have now developed a vaccination strategy that can create an army of T cells that are ready and waiting at those surfaces, offering a quicker response to viral invaders.
The researchers showed that they could induce a strong memory T cell response in the lungs of mice by giving them a vaccine modified to bind to a protein naturally present in mucus. This can help ferry the vaccine across mucosal barriers, such as the lining of the lungs… Continue reading.
Viruses Mutate, But Treatments Are Static. Is There a Way to Change That?
Leor Weinberger | Via UCSF | March 11, 2021There is a big, global problem: viruses such as HIV and COVID-19 mutate, but treatments for them don’t.
For more than 20 years, Leor Weinberger, PhD, has been thinking about how to make vaccines work more efficiently by being adaptive, rather than static.
“We’re fighting biology with chemistry,” said Weinberger, director of the Gladstone Center for Cell Circuitry and a professor in the Departments of Pharmaceutical Chemistry and Biochemistry and Biophysics at UC San Francisco. “Biology is dynamic, so it evolves. It transmits. Chemistry does neither of those things. It’s static… Continue reading.
PAGER-CoV: a comprehensive collection of pathways, annotated gene-lists, and gene signatures for coronavirus disease studies
Jake Chen | Via University of Alabama at Birmingham | March 10, 2021Jake Chen, Ph.D., chief bioinformatics officer at UAB Informatics Institute, is the latest winner of the School of Medicine’s Featured Discovery. This initiative celebrates important research from School of Medicine faculty members.
Chen and colleagues’ paper, “PAGER-CoV: a comprehensive collection of pathways, annotated gene-lists, and gene signatures for coronavirus disease studies,” was recently published in Nucleic Acids Research.
PAGER-CoV is a web-based database curated specifically for COVID-19 functional genomics research. The cutting-edge database holds nearly 12,000 pieces of genetic information called PAGs, which stand for “pathways, annotated gene-lists, and gene-signatures,” 1,549 candidate drug targets, and almost 20 million PAG-to-PAG association relationships on SARS-CoV-2… Continue reading.
New Technique Developed for Large-Scale Continuous Protein Purification
Andrew Zydney | Via Genetic Engineering & Biotechnology News | February 17, 2021Scientists have developed a new technique for purifying proteins during antibody manufacturing. The aim is to reduce the cost of continuous manufacturing for large-scale applications, such as COVID-19 therapeutics or Alzheimer’s disease treatments, without the need for Protein A.
“Protein A chromatography is robust and works well, but it’s also expensive and can create significant supply chain problems because you have to produce the Protein A and immobilize it on a resin,” says Andrew Zydney, PhD, professor of chemical engineering at Pennsylvania State University. “Our hope is that, by moving away from Protein A, we open up opportunities that are higher throughput and lower cost… Continue reading.
Researchers unravel what makes someone a COVID-19 super-spreader
David Edwards | Via Tulane University | February 9, 2021Scientists and public health experts have long known that certain individuals, termed “super-spreaders,” can transmit COVID-19 with incredible efficiency and devastating consequences.
Now, researchers at Tulane University, Harvard University, MIT and Massachusetts General Hospital have learned that obesity, age and COVID-19 infection correlate with a propensity to breathe out more respiratory droplets — key spreaders of SARS-CoV-2, the virus that causes COVID-19. Their findings were published in Proceedings of the National Academy of Sciences… Continue reading.
A UT Southwestern Machine Learning Algorithm Finds COVID-19 Cases Significantly Higher Than Reported
Gaudenz Danuser | Via Dallas Innovates | February 8, 2021Researchers in the Lyda Hill Department of Bioinformatics have estimated that the amount of COVID-19 cases is nearly triple those confirmed in the U.S.
While it has long been speculated that the number of COVID-19 cases is significantly higher than those reported, a new machine learning algorithm created by UT Southwestern Medical Center researchers from the Lyda Hill Department of Bioinformatics confirms this theory.
According to the algorithm, over 71 million people in the U.S. have contracted the virus. This number is almost three times as much as the 26.7 million publicly-reported number of confirmed cases, according to Jungsik Noh, Ph.D., a UT Southwestern assistant professor in the Lyda Hill Department of Bioinformatics… Continue reading.
Protein biosensors show promise for SARS-CoV-2 testing
David Baker | Via Science Board | February 1, 2021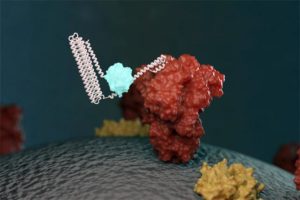
An illustration of a new biosensor binding to a targeted molecule and emitting light. Image courtesy of Ian Haydon/Institute for Protein Design at the University of Washington.
Scientists have developed biosensors to detect SARS-CoV-2 proteins and antibodies in simulated nasal fluids and human sera, according to a study published in Nature on January 27. The approach promises to be less costly and time-consuming than current COVID-19 testing methods.
Biosensors are devices used to detect the presence or concentration of specific biomolecules or biological structures. In this case, the researchers designed protein-based biosensors that recognize specific molecules on the surface of a particular virus and bind to them, then emit light through a biochemical reaction.
The scientists applied this approach to design biosensors of antibodies against SARS-CoV-2 protein epitopes and of the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein.
The result was a biosensor that glows when mixed with COVID-19 antibodies… Continue reading.
Algorithms Designed to Study Language Can Predict Immune “Escape” Mutations for HIV, Influenza, and SARS-CoV-2
Bonnie Berger | Via Genetic Engineering & Biotechnology News | January 15, 2021By bridging the conceptual divide between human language and viral evolution, MIT researchers have developed a powerful new computational tool for predicting the mutations that allow viruses to “escape” human immunity or vaccines. Its use could negate the need for high-throughput experimental techniques that are currently employed to identify potential mutations that could allow a virus to escape recognition. The computational model, based on models that were originally developed to analyze language, can predict which sections of viral surface proteins are more likely to mutate in a way that would enable viral escape, and it can also identify sections that are less likely to mutate, which would represent good targets for new vaccines.
“Viral escape is a big problem,” said Bonnie Berger, PhD, the Simons Professor of Mathematics and head of the Computation and Biology group at the Massachusetts Institute of Technology (MIT) Computer Science and Artificial Intelligence Laboratory. “Viral escape of the surface protein of influenza and the envelope surface protein of HIV are both highly responsible for the fact that we don’t have a universal flu vaccine, nor do we have a vaccine for HIV, both of which cause hundreds of thousands of deaths a year… Continue reading.
COVID’s Collateral Damage: Germicidal UV Lamps Can Damage Corneas
Jean-Marie Parel | Via Scitech Daily | January 7, 2021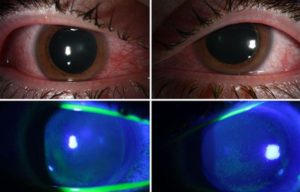
Injected conjunctiva (redness) of the right and left eye (top row) Diffuse staining of the cornea with green dye indicating epithelial damage (bottom row). Credit: Bascom Palmer Eye Institute
In a paper published in the journal of Ocular Immunology and Inflammation, physicians from the Bascom Palmer Eye Institute at the University of Miami Miller School of Medicine reported that several patients using germicidal lamps in an attempt to sanitize against the coronavirus, developed painful inflammation of the cornea, a condition called photokeratitis. These consumer-available ultraviolet (UV) emitting devices were being used in an attempt to eliminate coronavirus from homes and offices.
“During the height of the pandemic, we noticed an increased number of patients coming in with irritation, pain and sensitivity to light,” said first author and Bascom Palmer resident Jesse Sengillo, M.D. “We realized this was after direct exposure to germicidal lamps that emit UV light in the C range to kill bacteria and viruses. This can be quite a painful experience for the patient, but with prompt topical lubrication and antibiotics to prevent infection, patients often do very well… Continue reading.
Coronavirus likely infects upper airway cells first; blood pressure drugs unlikely to increase risk
Garry Nolan | Via Stanford Medicine | December 7, 2020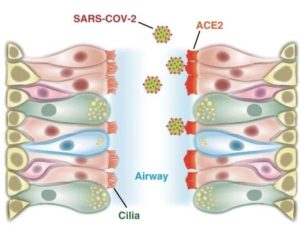
The coronavirus that causes COVID-19 binds to a protein on cells called ACE2, and researchers found high levels of ACE2 in airway cilia. Tsuguhisa Nakayama
Cells in the nasal passages and upper airways are likely the coronavirus’ major point of entry into the body, according to a study by Stanford Medicine researchers.
The finding further supports the use of masks to prevent viral spread and suggests that nasal sprays or rinses might be effective in blocking infection by the coronavirus.
The study also found that common blood pressure medications are unlikely to increase the risk of contracting COVID-19, countering concerns that hypertension drugs could make it easier for the coronavirus to enter human cells… Continue reading.
New CRISPR-based test for COVID-19 uses a smartphone camera
Daniel Fletcher | Via Science Daily | December 4, 2020Imagine swabbing your nostrils, putting the swab in a device, and getting a read-out on your phone in 15 to 30 minutes that tells you if you are infected with the COVID-19 virus. This has been the vision for a team of scientists at Gladstone Institutes, University of California, Berkeley (UC Berkeley), and University of California, San Francisco (UCSF). And now, they report a scientific breakthrough that brings them closer to making this vision a reality.
One of the major hurdles to combating the COVID-19 pandemic and fully reopening communities across the country is the availability of mass rapid testing. Knowing who is infected would provide valuable insights about the potential spread and threat of the virus for policymakers and citizens alike.
Yet, people must often wait several days for their results, or even longer when there is a backlog in processing lab tests. And, the situation is worsened by the fact that most infected people have mild or no symptoms, yet still carry and spread the virus… Continue reading.
Bahram Javidi Developing Rapid COVID-19 Testing
Bahram Javidi | Via University of Connecticut | December 3, 2020The COVID-19 pandemic has caused rapid changes across the globe in nearly every facet of life. Now, University of Connecticut professor of engineering Bahram Javidi is developing a low-cost, portable COVID-19 testing instrument to test for the virus just as quickly.
Through support from the Office of the Vice President for Research’s COVID-19 Research Seed Funding (COVID-RSF2) Program, Javidi is developing a novel technology with the potential to improve COVID-19 testing capacities. Javidi has appointments in the School of Engineering’s Departments of Electrical and Computer Engineering and Biomedical Engineering… Continue reading.
Using a ‘lab-on-a-chip’ in the fight against COVID-19
Jie Chen | Via Alberta Innovates | November 23, 2020Powerhouse researchers at the University of Alberta have joined forces to develop a handheld Lab-on-a-Chip (LOC) device for rapidly detecting COVID-19 antibodies. LOC technology involves the miniaturization and integration of components (e.g., sensors, a pump, a centrifuge, a microchip, etc.) into small, portable devices that can carry out laboratory tests whenever and wherever needed. Alberta researchers are at the forefront of advancing LOC technology and applying it to the detection of different diseases.
Project Title: Development and Clinical Validation of a Rapid Antibody Detection Device for COVID-19
Grant Amount: $304,200
Interdisciplinary Team:
- Jie Chen, Faculty of Engineering
- Jamil Kanji, Faculty of Medicine
- David Wishart, Faculty of Science
- Shawn Babiuk, Canadian Food Inspection Agency
This project funded by Alberta Innovates is led by Prof. Jie Chen. He and his team are on track to have a device prototype ready early next year that can quantify the concentration of COVID-19 antibodies in a droplet of blood quickly and accurately… Continue reading.
FDA Authorizes First Prescription At Home Molecular Test for COVID-19
Erik Engelson | Via Lucira Health | November 18, 2020 On November 18, 2020, the U.S. Food and Drug Administration (FDA) authorized the first prescription molecular diagnostic test for COVID-19 that can be performed entirely at home. The FDA issued an Emergency Use Authorization (EUA) to Lucira Health, Inc. for its single-use, user-friendly COVID-19 All-In-One Test Kit that can produce a positive or negative result at home within 30 minutes. Lucira’s test kit is differentiated by its simple ‘swab, stir and detect’ design. Clinical trials showed 100% of patients were successfully able to perform the Lucira test in about two minutes. That is significantly faster than labs which currently take two to seven days to generate similarly accurate test results.
On November 18, 2020, the U.S. Food and Drug Administration (FDA) authorized the first prescription molecular diagnostic test for COVID-19 that can be performed entirely at home. The FDA issued an Emergency Use Authorization (EUA) to Lucira Health, Inc. for its single-use, user-friendly COVID-19 All-In-One Test Kit that can produce a positive or negative result at home within 30 minutes. Lucira’s test kit is differentiated by its simple ‘swab, stir and detect’ design. Clinical trials showed 100% of patients were successfully able to perform the Lucira test in about two minutes. That is significantly faster than labs which currently take two to seven days to generate similarly accurate test results.
The Lucira™ COVID-19 All-In-One Test Kit is expected to be available to patients served by Sutter Health in Northern California, and Cleveland Clinic Florida in Miami-Ft. Lauderdale, in the near future. By early spring 2021, it is expected to be available nationally through health care providers. “There are currently two types of COVID-19 tests that detect whether a person is infected and potentially infectious,” said Lucira Health CEO Erik Engelson. “Antigen tests detect viral proteins and can provide results quickly. However, they are not diagnostically definitive and are more likely to miss an active coronavirus infection, or positive result, compared to molecular tests. Molecular tests like Lucira’s are 50 to 60 times more sensitive than antigen tests, and considered the ‘gold standard’ for determining if someone is infected… Continue reading.
Researchers find more precise way to detect pneumonia caused by COVID-19
Guang-Hong Chen | Via Wis Business | October 16, 2020Using cutting-edge artificial intelligence technology, UW‒Madison investigators have developed a far more precise way to identify cases of COVID-19 induced pneumonia.
Using a custom artificial intelligence algorithm called CV19-Net, the UW research team dug into a vast resource database of tens of thousands of COVID-19 chest X-rays to show its method could identify pneumonia caused by COVID-19 at a sensitivity of 88%, according to Guang-Hong Chen, PhD, professor of medical physics and radiology at the University of Wisconsin School of Medicine and Public Health.
From the tens of thousands of X-rays available, the team pared down the number of X-ray images to train the artificial intelligence algorithm and then evaluated the performance of the CV19-Net algorithm over 5,900 X-rays from approximately 3,000 patients between Feb. 1 and May 3, 2020… Continue reading.
Skin-care product based on U of T Engineering research donated to health-care workers fighting COVID-19
Milica Radisic | Via University of Toronto | October 13, 2020 A U of T Engineering spinoff company has donated its entire stock of skin-care product to health-care workers fighting the global pandemic.
A U of T Engineering spinoff company has donated its entire stock of skin-care product to health-care workers fighting the global pandemic.
Several years ago, Professor Milica Radisic (BME, ChemE) and her team developed a peptide-hydrogel biomaterial that prompts skin cells to “crawl” toward one another. The material was initially designed to help close the chronic, non-healing wounds often associated with diabetes, such as bed sores and foot ulcers.
Shortly thereafter, the technology was spun out into Quthero, Inc. a company with offices in Toronto and Pinecrest, Fla. Their first product, Kerra, incorporates the peptides designed by Radisic and her team, and is bioengineered to protect skin from everyday environmental stresses… Continue reading.
Team receives $4 million NIH grant for rapid test of COVID-19, other respiratory infections
Frederick Haselton | Via Vanderbilt University | October 13, 2020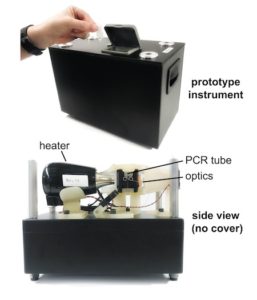 Twice in 2019, Nick Adams and his colleagues applied for federal grant money to develop a rapid, precise, in-office test for respiratory infections. This test would skip the time-consuming and expensive steps of purifying the samples for testing or sending them to a lab. Doctors and their patients would not have to wait days, sometimes weeks for results.
Twice in 2019, Nick Adams and his colleagues applied for federal grant money to develop a rapid, precise, in-office test for respiratory infections. This test would skip the time-consuming and expensive steps of purifying the samples for testing or sending them to a lab. Doctors and their patients would not have to wait days, sometimes weeks for results.
Their proposal got high marks for innovation. But the reviewing panel at the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, questioned its significance. Among the comments: Existing tests worked fine for diagnosing seasonal flu and pneumonia. What’s wrong with sending samples to a lab?
What a difference a year makes.
In June 2020 the reviewers were more receptive, and in September the NIAID awarded the team a five-year grant of nearly $4 million to develop a panel of quick tests to diagnose COVID-19 infections, seasonal flu and other respiratory illnesses. Adams, research assistant professor of Biomedical Engineering, had updated the application with information about the COVID-19 pandemic, shortages of testing components and an anticipated surge in demand for more widespread, frequent testing.
“Despite the initial funding rejections, we are fortunate to have continued to work toward developing a better test for respiratory illnesses,” Adams said. “We knew we had a good idea, but I guess we just had to wait for others to recognize it… Continue reading.
Northeastern University studying nanotechnology to help curb COVID-19 spread
Tom Webster | Via WCVB ABC | October 13, 2020A group of scientists at Northeastern University are making progress using nanotechnology to prevent, diagnose and fight the coronavirus.
Thomas Webster, professor of chemical engineering at Northeastern University, has been working with nanotechnology for decades. Now, he and his team are finding new applications with the coronavirus.
Their goal is to find ways to keep the virus from spreading, improve testing, and create a therapy. “This is why viruses are such a huge problem, because they’re so small and pervasive… Find out more.
Vanderbilt performs world’s first heart, lung transplant of COVID-19 patient
Matthew Bacchetta | Via News Channel 5 Nashville | October 9, 2020Vanderbilt University Medical Center says it performed the world’s first dual heart-lung transplant of a COVID-19 patient in September.
Vanderbilt says the patient, described as a young man, had cardiomyopathy – a disease of the heart tissue that can lead to heart failure – before he contracted COVID-19 in June.
The procedure, which was completed on Sept. 24, was also Vanderbilt’s first heart-lung transplant since 2006. Ashish Shah, MD, professor and chair of Cardiac Surgery, performed the complex surgery, along with Matthew Bacchetta, MD, MBA, associate professor of Thoracic Surgery… Continue reading.
Vanderbilt researchers develop publicly available COVID-19 animal susceptibility prediction tool; suggests increased risk to horses
John Wikswo | Via Vanderbilt University | October 6, 2020A Vanderbilt team of experts in virology, genetics, structural biology, chemistry, physiology, medicine, immunology and pharmacology have together developed technology to understand and predict animal susceptibility to SARS-CoV-2, the scientific name for the strain of coronavirus causing COVID-19. providing evidence that horses and camels may be at increased risk of the virus. The group has also released a publicly available tool to enable people to understand the likelihood of other animals’ susceptibility.
The article, “Predicting susceptibility to SARS-CoV-2 infection based on structural differences in ACE2 across species,” was published in the Federation of American Societies for Experimental Biology (FASEB) Journal on Oct. 5.
The investigators applied a combination of sophisticated genetic sequence alignment and structural analysis of ACE2, the receptor protein for SARS-CoV-2, to a variety of known susceptible and non-susceptible species. Through the analysis they identified five particular amino acid sites within the protein that distinguish virus susceptibility or resistance, and using these sites developed an algorithm to predict susceptibility of unknown species. The algorithm has been made public on a website where people can upload the aligned ACE2 sequence of animals with unknown susceptibility to generate a COVID-19 susceptibility score… Continue reading.
Wyss Institute’s nasal swab and toehold switch technologies licensed to facilitate SARS-CoV-2 diagnostic efforts
Donald Ingber | Via Harvard University | October 5, 2020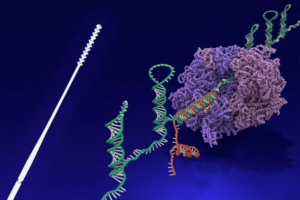
The Wyss Institute for Biologically Inspired Engineering at Harvard University announced today that its nasopharyngeal swab and toehold switch technologies have been licensed to Alabama-based Agile Biodetection, which will use them to develop solutions for unmet diagnostic needs in the detection of the SARS-CoV-2 virus in environmental or clinical settings. The licensing agreement was coordinated by Harvard’s Office of Technology Development (OTD) in accordance with the University’s commitment to the COVID-19 Technology Access Framework.
The Wyss Institute’s nasopharyngeal (nasal) swabs were developed in a multi-institutional and multi-disciplinary group effort led by Wyss Institute Senior Staff Engineer Richard Novak, Ph.D., and the Wyss’ Founding Director Donald Ingber, M.D., Ph.D., as part of the Institute’s collective response to the COVID-19 pandemic. Motivated by the serious shortage of swab devices for the collection of nasopharyngeal samples early in the pandemic, the researchers created a simple and effective device with advantages over other designs. The Wyss swab device is fully injection-molded from a single material, and as such, can be mass manufactured in a one-step process that is faster, less expensive, and routinely used by a broad range of experienced medical device manufacturers worldwide. Conventional nasal swabs that are commonly used in infectious disease diagnostic medicine were designed 50 years ago, and are manufactured in two parts from different materials that then need to be assembled, sterilized and packaged in a multi-step process, which requires considerable time and expense. In successful tests performed by academic collaborators and teaching hospitals, the unique nasal swab design was demonstrated to effectively collect SARS-CoV-2 genetic RNA material from the nostrils of patients and to be more comfortable than existing commercial products… Continue reading.
COVID test site differences, a fourth option in the works
Rebecca Richards-Kortum | Via Rice University | September 24, 2020Rice’s Crisis Management Team plans to add a fourth and more rapid COVID-19 testing option on the Rice campus. Currently there are three sites that provide daily testing for asymptomatic students, staff and faculty who spend time on campus.
All three of these current sites (Abercrombie Engineering Laboratory, East Gym in the Tudor Fieldhouse and The Roost at Reckling Park) offer polymerase chain reaction testing. Bioengineering professor Rebecca Richards-Kortum said that her lab is working with the MD Anderson Cancer Center to develop a nucleic acid test for the fourth testing option… Continue reading.
Introducing COVID19questions.org
Lucila Ohno-Machado | Via UC San Diego Health | September 17, 2020 As the COVID-19 pandemic continues, there is an urgent need to determine who is at greatest risk for severe disease, better understand how the disease and treatments evolve, and predict the need for resources. But to get there, researchers and clinicians need more data about what patients have experienced so far, and what factors are associated with different patient outcomes.
As the COVID-19 pandemic continues, there is an urgent need to determine who is at greatest risk for severe disease, better understand how the disease and treatments evolve, and predict the need for resources. But to get there, researchers and clinicians need more data about what patients have experienced so far, and what factors are associated with different patient outcomes.
To provide this information, a new research consortium invites clinicians, researchers, patients and the general public to submit questions that could be answered by COVID-19 patient record data from more than 200 participating hospitals. Questions are submitted and answers are provided via a new web portal: COVID19questions.org.
The consortium, called Reliable Response Data Discovery (R2D2), is led by Lucila Ohno-Machado, MD, PhD, chair of the Department of Biomedical Informatics at UC San Diego Health, and made possible by seed funding from the Gordon and Betty Moore Foundation. R2D2 comprises 12 health systems (202 hospitals) to date: UC San Diego Health, Cedars Sinai Medical Center, Ludwig Maximilian University of Munich, San Mateo Medical Center, UC Davis Health, UC Irvine, UCLA, UCSF, University of Colorado Anschutz Medical Campus, University of Southern California, University of Texas Health Science Center at Houston and Memorial Hermann Health System, and the Veterans Affairs (VA) Healthcare System… Continue reading.
New Insights into How COVID-19 Causes Heart Damage
Todd McDevitt | Via Gladstone Institutes | August 25, 2020COVID-19 was initially identified as a respiratory disease, but scientists now appreciate that it also affects several other organs in the body, including the heart. Heart damage is a major determinant of COVID-19 related deaths, and even patients who experience only mild COVID-19 symptoms exhibit signs of cardiac dysfunction several months after recovery.
A new study by scientists at Gladstone Institutes helps explain how SARS-CoV-2, the virus that causes COVID-19, inflicts damage on heart cells. The team’s findings, shared publicly on bioRxiv, show the virus’s unexpected effects on the structure of heart cells in the lab, as well as in heart tissue from COVID-19 patients.
The team, led by Gladstone Senior Investigators Todd C. McDevitt, PhD, and Bruce R. Conklin, MD, was uniquely positioned to tackle this work, due to their experience in deriving various types of cardiac cells in the lab from induced pluripotent stem cells… Continue reading.
Research Examines Links Between ‘Long COVID’ and ME/CFS
Ronald Tompkins | Via Medscape | August 24, 2020The persistence of long-term symptoms in some individuals with COVID-19 illness has opened up a new line of research into the mechanisms underlying myalgic encephalomyelitis / chronic fatigue syndrome and other chronic post-viral illnesses.
Some patients who had COVID-19 continue to have symptoms weeks to months later, even after they no longer test positive for the virus. In two recent reports — one published in the Journal of the American Medical Association in July and another published in Morbidity and Mortality Weekly Report this month — chronic fatigue was listed as the top symptom among individuals still feeling unwell beyond 2 weeks after COVID-19 onset… Continue reading.
New Vaccine Platform – With No Needles – Has Potential to Be More Effective With Fewer Side Effects
Joel Collier | Via SciTech Daily | August 7, 2020Study shows that peptide nanofibers induce immune response in lungs and lymph nodes without requiring adjuvants for efficacy, indicating promise for new vaccine development.
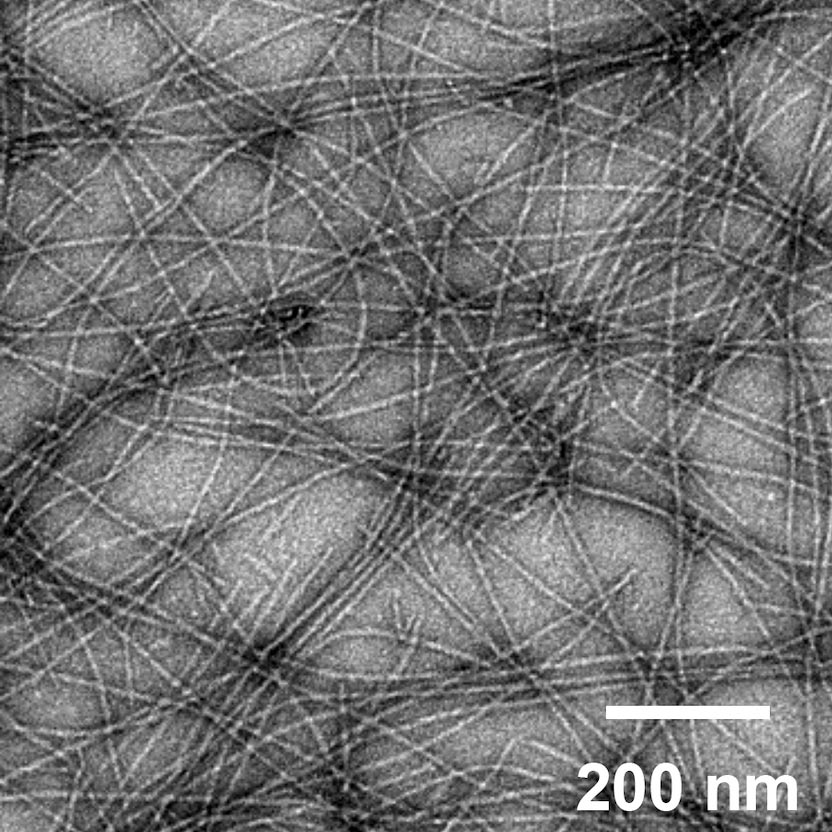
An image of self-assembled peptide nanofibers, which are currently under investigation for engineered vaccines. Credit: Collier Lab
The ongoing COVID-19 pandemic is shining a bright spotlight on vaccine development. As numerous vaccines race through clinical trials, physicians and researchers continue to work on developing new vaccine technologies to generate the most effective vaccines with the fewest side effects.
A new proof-of-concept study by researchers at the University of Chicago and Duke University demonstrates the potential for one such platform, using self-assembling peptide nanofibers tagged with antigens to prime the immune system against a potential invasion.
Their research, published in Science Advances on August 7, 2020, showed that these nanofibers can induce an immune response and activate T cells without the use of additional adjuvants, which can induce inflammation and are associated with common vaccine side effects, like soreness at the injection site or low-grade fever… Continue reading.
UChicago awarded $20 million to host COVID-19 medical imaging center
Maryellen Giger | Via University of Chicago | August 7, 2020Two-year federal contract will support open-source database, enable AI-driven research
A new center hosted at the University of Chicago—co-led by the largest medical imaging professional organizations in the country—will help tackle the ongoing COVID-19 pandemic by curating a massive database of medical images to help better understand and treat the disease.
Led by Prof. Maryellen Giger of UChicago Medicine, the Medical Imaging and Data Resource Center (MIDRC) will create an open-source database with medical images from thousands of COVID-19 patients. The center will be funded by a two-year, $20 million contract from the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health (NIH)… Continue reading.
Rapid antibody development yields possible treatment for yellow fever
Ram Sasisekharan | Via MIT | July 29, 2020Yellow fever, a hemorrhagic disease that is common in South America and sub-Saharan Africa, infects about 200,000 people per year and causes an estimated 30,000 deaths. While there is a vaccine for yellow fever, it can’t be given to some people because of the risk of side effects, and there are no approved treatments for the disease.
An international team of researchers, led by MIT Professor Ram Sasisekharan, has now developed a potential treatment for yellow fever. Their drug, an engineered monoclonal antibody that targets the virus, has shown success in early-stage clinical trials in Singapore.
This class of antibodies holds promise for treating a variety of infectious diseases, but it usually takes several years to develop and test them. The MIT-led researchers demonstrated that they could design, produce, and begin clinical trials of their antibody drug within seven months… Continue reading.
UArizona Aims to Provide Rapid Tests for Exposure to Biothreats, Including COVID-19
Frederic Zenhausern | Via University of Arizona | July 27, 2020Thanks to a U.S. Department of Defense contract for as much as $9.5 million, the University of Arizona College of Medicine – Phoenix and partners aim to develop a portable device to easily and accurately detects biological threats, including the virus that causes COVID-19.
To better protect those serving on the front lines of battlefields or dealing with an event like the COVID-19 health crisis or potential future pandemics, scientists at the University of Arizona College of Medicine – Phoenix are leading an effort to develop a device that could easily, quickly and accurately detect pathogens and biological threats.
The college’s Center for Applied Nanobioscience and Medicine is leading the effort, under an Other Transaction Agreement with the Defense Threat Reduction Agency, an agency within the U.S. Department of Defense. The contract, to provide about 3,000 devices, has a $9.5 million ceiling for three years.
Frederic Zenhausern, the center’s director and interim co-chair of the Department of Basic Medical Sciences, and his team members, including associate professor Jian Gu, are creating the device in collaboration with scientists from the University of Nevada, Arizona State University, Duke University and Whitespace Enterprises, an Arizona-based startup founded by Zenhausern. The group is responsible for mass production of the technology… Continue reading.
In Cell Studies, Seaweed Extract Outperforms Remdesivir in Blocking COVID-19 Virus
Jonathan Dordick | Via News Wise | July 24, 2020Heparin, a common anitcoagulent, could also form basis of a viral trap for SARS-CoV-2
In a test of antiviral effectiveness against the virus that causes COVID-19, an extract from edible seaweeds substantially outperformed remdesivir, the current standard antiviral used to combat the disease. Heparin, a common blood thinner, and a heparin variant stripped of its anticoagulant properties, performed on par with remdesivir in inhibiting SARS-CoV-2 infection in mammalian cells.
Published online today in Cell Discovery, the research is the latest example of a decoy strategy researchers from the Center for Biotechnology and Interdisciplinary Studies (CBIS) at Rensselear Polytechnic Institute are developing against viruses like the novel coronavirus that spawned the current global health crisis… Continue reading.
AIMBE Fellows’ Rapid and Ongoing Global Clinical Engineering Response to COVID-19
Tom Judd | Via AIMBE | July 23, 2020With Yadin David, and Elliot Sloane, IFMBE Clinical Engineering Division (CED)
Situation
Three AIMBE Fellows created and lead an extensive global COVID-19 (C19) information and training program. The global Coronavirus pandemic (C19) is a crisis that demands agile worldwide response, not only by countries and their health systems, but also by most individuals on the planet. This has been a time of incredible suffering, of new challenges, and also unprecedented opportunity for the clinical engineering (CE)/health technology (HT) global community to come together and share experiences – as well as learned and improvised best practices – for medical device management and related tools used to address the prevention, diagnosis, and treatment of C19. CED provides this information at no charge to its global network of over 15,000 CE/HT colleagues.
Background
In response to this crisis and the World Health Organization (WHO’s) request in March 2020, three AIMBE Fellows launched a program of free global webinars and information tools sharing clinical engineering (CE) learnings related to the COVID19 pandemic… Continue reading.
This COVID-19 super antibody test could provide new insights into immunity
David Walt | Via Fast Company | July 21, 2020COVID-19 antibody tests have been the subject of scrutiny since their arrival, but they still represent an important tool in understanding population health. Molecular tests have become the top method of identifying cases of COVID-19. One scientist thinks we should be looking at using a combination of antibody tests, antigen tests, and molecular RNA tests to better understand who has COVID-19 and whether or not they’re actively recovering.
Dr. David Walt is one of the cofounders of genetic sequencing technology giant Illumina and Quanterix, a company that makes technology for detecting biomarkers. He is also co-director of the MGB Center for COVID Innovation at Brigham and Women’s Hospital, in Boston. When COVID-19 struck, he had to close his lab at Harvard University due to the pandemic. He petitioned to reopen, so he and a team could work on a super antibody test that would enable him to better understand immune response in COVID-19 patients. The request was approved… Continue reading.
Paige Receives FDA Clearance for the FullFocus Viewer for Digital Pathology
Leo Grady | Via Business Wire | July 21, 2020Paige, a global leader in computational pathology, today announced it received Federal Drug Administration (FDA) 510(k) clearance for the FullFocus™, a digital pathology image viewer for the purpose of primary diagnosis. This 510(k) clearance from the FDA allows in vitro diagnostic (IVD) use of FullFocus with FDA-authorized Philips Ultra Fast Scanner and paves the way for IVD use of FullFocus with additional IVD Whole Slide Imaging (WSI) scanners in the future.
Lack of interoperability, intensive capital requirements and burdensome, on-premise storage have long been a challenge in the adoption of digital pathology. The foundation for the FullFocus viewer was initially created and validated at Memorial Sloan Kettering Cancer Center (MSK) to allow researchers and pathologists to intuitively view and navigate digital images of surgical pathology slides acquired on all major commercial brands of WSI scanners. After refinement based on 18 months of daily use for retrospective slide review by dozens of practicing pathologists at MSK, the viewer was further enhanced by Paige to meet the performance requirements for IVD use, with accurate color reproducibility, optimized viewing speeds and adherence to a certified quality management system. Committed to providing a flexible solution for hospitals, Paige is working to expand upon the clearance to incorporate use with additional WSI scanners and monitor displays in the near future… Continue reading.
COVID-19 vaccine development and a potential nanomaterial path forward
Nicole Steinmetz | Via Nature Nanotechnology | July 15, 2020Abstract
The COVID-19 pandemic has infected millions of people with no clear signs of abatement owing to the high prevalence, long incubation period and lack of established treatments or vaccines. Vaccines are the most promising solution to mitigate new viral strains. The genome sequence and protein structure of the 2019-novel coronavirus (nCoV or SARS-CoV-2) were made available in record time, allowing the development of inactivated or attenuated viral vaccines along with subunit vaccines for prophylaxis and treatment. Nanotechnology benefits modern vaccine design since nanomaterials are ideal for antigen delivery, as adjuvants, and as mimics of viral structures. In fact, the first vaccine candidate launched into clinical trials is an mRNA vaccine delivered via lipid nanoparticles. To eradicate pandemics, present and future, a successful vaccine platform must enable rapid discovery, scalable manufacturing and global distribution. Here, we review current approaches to COVID-19 vaccine development and highlight the role of nanotechnology and advanced manufacturing… Continue reading.
David Edwards develops a $50 nasal spray to thwart the spread of COVID-19
David Edwards | Via Fast Company | July 8, 2020Developed by Harvard professor David Edwards—who previously created inhalable chocolate and cocktails—the spray promises to stop the spread of aerosols by as much as 99%.
 You should practice social distancing and wear a mask to prevent the spread of COVID-19. But even masks aren’t perfect. A high-end N95 mask can filter an estimated 99.8% of the virus from the air, while many cotton masks filter just 50% or less. Given that researchers now know the virus is airborne, you may wonder: Is there anything else you can do to prevent the spread of COVID-19?
You should practice social distancing and wear a mask to prevent the spread of COVID-19. But even masks aren’t perfect. A high-end N95 mask can filter an estimated 99.8% of the virus from the air, while many cotton masks filter just 50% or less. Given that researchers now know the virus is airborne, you may wonder: Is there anything else you can do to prevent the spread of COVID-19?
According to David Edwards, a Harvard professor and entrepreneur, there is. And it’s not much more complicated than sniffing a specialized saline solution. “It’s cleaning my bioaerosol footprint, if you will,” he says.
With his company Sensory Cloud, Edwards has developed a $50 product that has two components: the Nimbus and FEND. The Nimbus is an aerosol squirter, capable of turning liquid into a cloud of vapor that you puff in front of your nose to inhale from the air. FEND is a solution that goes inside the squirter, composed of a mix of salts similar to seawater… Continue reading.
Encouraging results from functional MRI in an unresponsive patient with COVID-19
Bruce Rosen | Via Medical Xpress | July 6, 2020Many patients with severe coronavirus disease 2019 (COVID-19) remain unresponsive after surviving critical illness. Investigators led by a team at Massachusetts General Hospital (MGH) now describe a patient with severe COVID-19 who, despite prolonged unresponsiveness and structural brain abnormalities, demonstrated functionally intact brain connections and weeks later he recovered the ability to follow commands. The case, which is published in the Annals of Neurology, suggests that unresponsive patients with COVID-19 may have a better chance of recovery than expected.
In addition to performing standard brain imaging tests, the team took images of the patient’s brain with a technique called resting-state functional magnetic resonance imaging (rs-fMRI), which evaluates the connectivity of brain networks by measuring spontaneous oscillations of brain activity. The patient was a 47-year-old man who developed progressive respiratory failure, and despite intensive treatment, he fluctuated between coma and a minimally conscious state for several weeks… Continue reading.
How Credible are the COVID-19 Models? Center Aims to Find Out
Herbert Sauro | Via University of Washington | July 6, 2020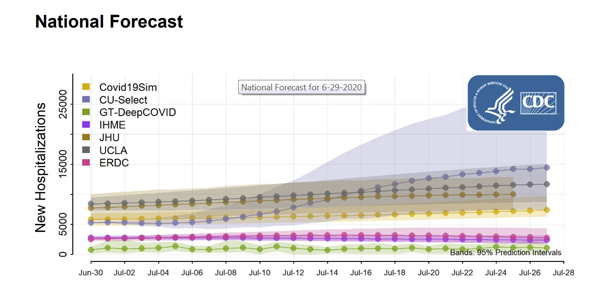
The multi-institution Center for Reproducible Biomedical Modeling, led by UW Bioengineering Professor Herbert Sauro, is partnering with top U.S. government agencies to determine how credible several commonly used COVID-19 models are.
Since the early days of the coronavirus pandemic, to aid in decision making, public health officials and policy makers have looked to epidemiological models that estimate how many people will likely get sick, need hospitalization and die from the new virus.
“Our role is to come up with a system that can be used to rank COVID-19 models in terms of reproducibility and credibility… Continue reading.
Dr. Cato T. Laurencin’s COVID-19 Mask Solution Coming to Market
Cato Laurencin | Via University of Connecticut | July 2, 2020Within six weeks of announcing a successful method to fabricate custom-fit mask frames to optimize protection from the spread of COVID-19, UConn has a licensing deal with a Connecticut manufacturer to produce them.
Connecticut Biotech, a startup company headquartered in South Windsor, aims to start marketing, manufacturing, and distributing 3D-printed mask frames under the brand Secure Fit this month.
“This is an important technology that can help a lot of people by providing a specific way to make regular surgical masks more protective,” says Dr. Cato T. Laurencin, CEO of the Connecticut Convergence Institute for Translation in Regenerative Engineering. “It’s wonderful to see technology that started here in the state of Connecticut being developed by a Connecticut company… Continue reading.
DNA Origami Approach for HIV Vaccine Development Could be Used Against SARS-CoV-2
Darrell Irvine | Via Clinical Omics | June 30, 2020By using a technique known as DNA origami to fold DNA into a virus-like structure, MIT researchers have designed HIV-like particles coated with HIV antigens in precise patterns, which may eventually be used as an HIV vaccine. In vitro studies showed that the DNA origami particles, which mimic the size and shape of viruses, provoked a strong immune response from human immune cells. The researchers anticipate that the same approach could be used to design DNA origami vaccines for a wide variety of viral diseases, and they are now working on adapting the technology to develop a potential vaccine for SARS-CoV-2.
“The rough design rules that are starting to come out of this work should be generically applicable across disease antigens and diseases,” said Darrell Irvine, PhD, who is the Underwood-Prescott professor with appointments in the departments of biological engineering and materials science and engineering; an associate director of MIT’s Koch Institute for Integrative Cancer Research; and a member of the Ragon Institute of MGH, MIT, and Harvard. “Our platform technology allows you to easily swap out different subunit antigens and peptides from different types of viruses to test whether they may potentially be functional as vaccines,” added Mark Bathe, PhD, an MIT professor of biological engineering and an associate member of the Broad Institute of MIT and Harvard… Continue reading.
Researchers bioengineer first-line defense against COVID-19
Mark Humayun | Via Medical Xpress | June 30, 2020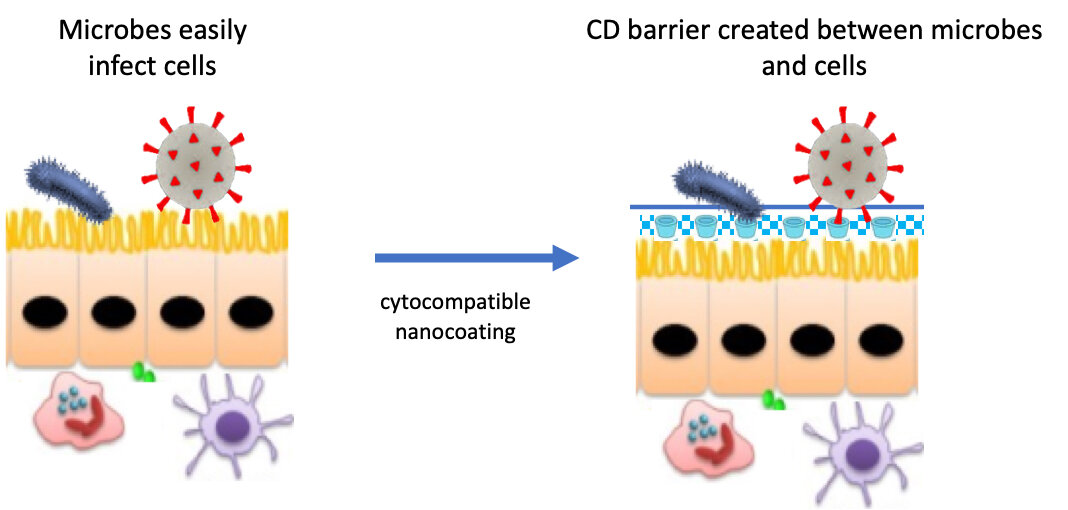
Researchers at the USC Dr. Allen and Charlotte Ginsburg Institute for Biomedical Therapeutics, the USC Institute for Technology and Medical Systems and the USC School of Pharmacy are developing an antimicrobial fluid to bolster the body’s first-line defenses against COVID-19.
The biocompatible coating is intended to block the virus from entering the body through membranes in the nose, eyes, and mouth. If successful, the invention could change the way medicine prevents certain infectious diseases.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the infectious agent that causes COVID-19, latches onto structures on the surface of our cells in order to invade. One of these membrane structures, known as a lipid raft, contains cholesterols and fats and acts as the subcellular equivalent of a dock at a seaport. Viral particles traversing our respiratory and gastrointestinal tracts hook onto lipid rafts, then enter our cells and use our cellular machinery to replicate… Continue reading.
Can “Nanosponges” Help Treat Patients With Coronavirus?
Liangfang Zhang | Via Forbes | June 17, 2020With news yesterday out of the UK that the inexpensive and widely available steroid dexamethasone significantly reduced deaths in coronavirus patients who are intubated and those requiring oxygen, following published evidence last month that the antiviral Remdesivir shortened time to recovery, the search for a breakthrough drug or approach that improves survival before approval of a viable vaccine remains illusive.
Add to this the potential for the virus to mutate—already with multiple strains— the search for a new approach would be ideal.
Now, researchers at UC San Diego have pioneered a novel pathway for treating infections using “nanosponges”—a technology that may hold promise for treating patients with SARS-CoV-2, the virus responsible for Covid-19… Continue reading.
Repurposing approved drugs for COVID-19 at an accelerated pace
Donald Ingber | Via Harvard University | June 16, 2020The United States’ Defense Advanced Research Projects Agency (DARPA) has signed an Agreement worth up to $16 million over the next year with the Wyss Institute for Biologically Inspired Engineering at Harvard University to identify and test FDA-approved drugs that could be repurposed to prevent or treat COVID-19. This highly collaborative effort leverages the Institute’s computational drug discovery pipelines and human Organ Chip technologies, and has already found multiple approved compounds that show promise against the SARS-CoV-2 virus that causes COVID-19.
The team, led by Wyss Founding Director Donald Ingber, M.D., Ph.D., is continuing to evaluate many more drugs, and lead compounds are being tested in high-throughput cell-based assays with CoV-2 virus in the lab of Matthew Frieman, Ph.D., Associate Professor of Microbiology and Immunology at the University of Maryland School of Medicine. The most promising drugs are being transferred to the lab of Benjamin tenOever, Ph.D. at the Icahn School of Medicine at Mount Sinai for testing in COVID-19 animal models. Human Organ Chip technology is also being set up in the Frieman and tenOever labs with equipment supplied by Wyss spinout Emulate, Inc., so that they can carry out experiments analyzing the human response to COVID-19 infection in vitro… Continue reading.
NSF RAPID grant supports COVID-19 ‘computational pipeline’
Lydia Kavraki | Via Rice University | June 16, 2020Lydia Kavraki, the Noah Harding Professor of Computer Science at Rice, has received a National Science Foundation (NSF) Rapid Response Research grant to implement a computational pipeline to help identify fragments of SARS-CoV-2 viral proteins that could be used as targets for vaccine development.
“Efforts are already underway to produce new drug inhibitors, repurpose existing drugs and devise combination treatments for COVID-19,” said Kavraki, who is also a professor of bioengineering, electrical and computer engineering and mechanical engineering… Continue reading.
Paul Yager developing rapid COVID-19 tests for the home and clinic
Paul Yager | Via University of Washington | June 11, 2020
A working, early prototype of the Yager lab’s screening device, which would allow users to test for COVID-19 at home.
At the onset of the COVID-19 pandemic, Paul Yager, professor of bioengineering, knew a rapid and accurate test would be needed to screen patients for the new coronavirus. He immediately set to work adapting his point-of-care testing research to developing an at-home test for the new virus.
Since 2011, his lab has been developing compact, low-cost screening tests that can detect illnesses such as flu, dengue fever and HIV, and can be used by anyone, anywhere. The Yager lab’s technology uses nucleic acid amplification tests (NAAT) coupled with microfluidic devices to detect fragments of a pathogen’s genetic material in under 20 minutes. It offers a simpler and faster alternative to traditional gene-based testing, which requires a complicated lab process that takes a few hours to get reliable results.
Now, the Yager lab is applying its technology, called UbiNAAT, to COVID-19 tests, which could be used by untrained people in their homes as well as in health care facilities and low-resource settings around the world. The tests could also be used for screening at airports, workplaces, theaters and sporting events to identify carriers of the virus and limit its spread… Continue reading.
How rod-shaped particles might distract an out-of-control COVID immune response
Lola Eniola-Adefeso | Via University of Michigan | June 10, 2020A long-ignored white blood cell may be central to the immune system overreaction that is the most common cause of death for COVID-19 patients—and University of Michigan researchers found that rod-shaped particles can take them out of circulation.
The No. 1 cause of death for COVID-19 patients echoes the way the 1918 influenza pandemic killed: their lungs fill with fluid and they essentially drown. This is called acute respiratory distress syndrome. But a new way of drawing immune cells out of the lungs might be able to prevent this outcome. This research is among the essential projects at U-M that have continued through the pandemic uninterrupted… Continue reading.
DoD Funds Clinical Trial of Seraph Blood Filter to Treat COVID-19
Robert Ward | Via MD+DI Online | June 9, 2020The Department of Defense is funding a clinical trial of a COVID-19 treatment using ExThera Medical’s Seraph 100 Blood filter.
The device was selected to be in the multi-center randomized clinical trial because it showed encouraging preliminary results in critically ill COVID-19 patients at a military hospital in the U.S. and 14 other hospitals in Europe. Investigators at the Uniformed Services University in Bethesda will run the trial of the Martinez, CA-based company’s device. Initial reports indicate Seraph 100 stabilizes blood pressure and inflammatory biomarkers that correlate with poor patient outcome: IL-6, Ferritin, D-dimers, LDH, and Nt-proBNP, all decreased during Seraph 100 treatments of COVID-19 patients.
In a release about DoD’s funding, it was stated that it appears as if the Seraph 100 helps improve patient outcomes by providing additional time for supportive care while reducing the sources of inflammation and possibly preventing further damage by reducing SARS-CoV-2 virus/RNA in the bloodstream… Continue reading.
Study Finds Screened Health Care Workers Unlikely to Be Covid-19 Carriers
Terence Sanger | Via MedicalResearch.com | June 4, 2020MedicalResearch.com Interview with:
 Dr. Terence Sanger MD
Dr. Terence Sanger MD
Pediatric Neurology
Vice president of
Research and Chief Scientific Officer
CHOC Children’s
UC Irvine School of Medicine
MedicalResearch.com: What is the background for this study?
Response: During the current pandemic, publicly available data on the prevalence of COVID-19 infection among healthcare workers has been limited. This study sought to determine the COVID-19 viral prevalence in a population of healthcare workers within a pediatric emergency department in Orange County, Calif., during a time interval that overlapped with the state’s projected peak coronavirus-related use of hospital resources.
MedicalResearch.com: What are the main findings?
Response: We found that the burden of COVID-19 disease, as measured by RT-PCR, was 0.69% (1/145) in our cohort of asymptomatic healthcare workers in a pediatric emergency department. Healthcare workers in a community with a low prevalence of COVID-19 who undergo daily symptom surveys and temperature screens are not likely to be carriers of Sars-CoV-2. These findings could help assuage public fear of contracting COVID-19 from emergency department providers… Continue reading.
Wearable COVID-19 Sensor Receives NSF RAPID Grant
John Rogers | Via Northwesternn University | June 3, 2020Device monitors early signs, disease progression and response to treatment
The skin mounted sensor is soft, flexible and comfortable to wear.
A research team led by Northwestern Engineering bioelectronics pioneer John A. Rogers has received a $200,000 grant from the National Science Foundation (NSF) to continue developing a novel wearable device and set of algorithms specifically tailored to catch early signs and monitor progression of COVID-19.
In partnership with researchers at Shirley Ryan AbilityLab, Rogers launched the device in April. The NSF funding will help Rogers and his team incorporate more advanced data analytics into the device and add a sensor to measure oxygen levels in the blood.
This project is among the latest at Northwestern to receive a rapid response research (RAPID) grant from the NSF, which has called for immediate proposals that have potential to address the spread of COVID-19.
“Our device addresses a key issue in the COVID-19 pandemic: the limited capacity of healthcare systems,” Rogers said. “By continuously monitoring high-risk individuals, such as healthcare workers and the elderly, we can minimize the number of unnecessary hospital visits and provide an early warning to enable preventive measures… Continue reading.
USF COVID-19 Rapid Response Research Effort Delivers Funding in Second Round of Pandemic Projects
Paul Sanberg | Via University of South Florida | May 29, 2020The University of South Florida’s COVID-19 Rapid Response Grants program is investing in 14 faculty research projects that would advance new medical interventions to detect and stop infections, develop innovations in personal protective equipment, and address fear and confusion in communities particularly vulnerable to the virus.
A total of $344,855 will support this new round of research projects — the second such investment made by the university since April. USF is partnering with the Florida High Tech Corridor Council which is contributing $100,000 in support of five of the proposals which have the potential for technology commercialization.
…
“The shock and devastation of the pandemic has inspired USF researchers to dig deep in thinking of new ways to keep us safer and healthier in the future,” said Dr. Paul R. Sanberg, USF’s Senior Vice President for Research, Innovation & Knowledge Enterprise. “They are applying the best of their expertise, ability and creativity to this cause, while working in partnership with private companies and public agencies to bring these projects to fruition… Continue reading.
Tampa company, Kaligia Biosciences, developing portable, rapid, saliva-based COVID-19 screening devices
Stephen Liggett | Via Tampa Dispatch | May 27, 2020The reopening of the world economy is largely reliant on easy and accessible COVID-19 screening. Kaligia Biosciences, a medical device company, is working with major Florida medical institutions to develop a portable, saliva-based device that can produce results in less than three minutes.
Kaligia Biosciences is starting clinical trials of the Rapid Biofluid Analyzer 2 (RBA-2) device this week, using saliva samples of COVID-19 from Bay-area hospitals and beyond.
The method uses a proven Kaligia Biosciences device that analyses multiple blood components. Kaligia Biosciences is collaborating with AdventHealth, the University of South Florida College of Pharmacy (USF) the USF College of Medicine and Tampa General Hospital (TGH) to adapt the device for COVID-19 screening… Continue reading.
Decreasing the Time from Antibody Idea to IND Approval
Ram Sasisekharan | Via Genetic Engineering & Biotechnology News | May 26, 2020In 2015, Ram Sasisekharan, PhD, the Alfred H. Caspary professor of biological engineering and health sciences & technology at MIT, founded Tychan. The company concentrates on one key goal: decreasing the time from antibody idea to investigational new drug (IND) approval. Now, the company claims it can cut the average time in half and more.
“With traditional technology, it usually takes about 18 months to get an antibody into human trials, from discovery to development,” Sasisekharan said. “For the Zika and Yellow Fever viruses, we developed new antibodies that went into human trials in less than nine months.” This new speed arises from a combination of informatics and bioprocessing… Continue reading.
Prophylactic Drug Delivery System for COVID-19
Heather Sheardown | Via McMaster University | May 22, 2020The Heather Sheardown lab (McMaster University, Canada) is home to an interdisciplinary team of scientists and trainees with expertise in ophthalmology, polymer and biomaterial engineering, chemistry, pharmaceutical formulation and drug delivery, animal/ex-vivo/in-vitro models of disease and drug delivery, early stage material design and synthesis, and synthetic method scalability optimization.
As the availability of a SARS-CoV-2 vaccine is still far off, there is an immediate global need for prophylactic prevention strategies, particularly for vulnerable populations including seniors and frontline workers. The Sheardown lab has developed a mucoadhesive polymeric micelle that allows for the encapsulation of a range of therapeutics, providing local, controlled delivery to mucosal surfaces. This technology overcomes traditional solubility concerns, allowing formulations at higher drug concentrations. Its mucosal binding significantly reduces dosing frequency, increases local bioavailability and improves clinical efficacy. Developed and validated for safety and efficacy in the eye, this system is now being repurposed for the mucosa of the respiratory tract, formulated as a nasal spray or inhaled aerosol, incorporating two treatments that are currently under study internationally: hydroxychloroquine (HCQ) and remdisivir… Continue reading.
Go behind the virus on its entry way with targeted pharmacological therapy: the inhalation route of anti SARS-Cov2 active substances.
Ruggero Bettini | Via AIMBE Public Documents | May 21, 2020BACKGROUND
SARS-Cov2 is the last appeared coronavirus that developed a pandemic infection with huge number of fatal cases. No vaccines are yet available that protect from this infection. However, a number of therapeutic tactics against COVID-19 have been empirically started.
According to Mehra et al., 2020, in COVID-19 illness, a structured approach to clinical is imperative (1). Such approach distinguish the phase in which the viral pathogenicity is dominant, from the phase where the host inflammatory response overtakes the pathology. Therefore, after five months of epidemy, 3-stage’s progression of illness, corresponding to increasing severity with distinct clinical signs, therapy responses and clinical outcomes, has been assessed. These stages constitute the reference for investigation and proposition of effective targeted therapies (1).
Stage I is the phase where the early infection causes to most people mild or asymptomatic disease. Treatment at this level with drug having antiviral activity could prevent the progression to severe disease. Stage II (moderate) corresponds to the pulmonary involvement without (IIa) and with (IIb) hypoxia. In this stage the pulmonary disease is established with viral lung multiplication and localized inflammation. The patients develop a viral pneumonia, with cough, fever and possibly hypoxia. Now, not only antiviral drugs are required but, in particular, the inflammation has to be considered and treated. Stage III is the phase in which an extra-pulmonary systemic hyperinflammation syndrome manifests. Likely, a minority of Covid-19 patients transit to this stage where the therapy is essentially against the so called “inflammation storm… Continue reading.
Go behind the virus on its entry way with targeted pharmacological therapy: the inhalation route of anti SARS-Cov2 active substances.
Paolo Colombo | Via AIMBE Public Documents | May 21, 2020BACKGROUND
SARS-Cov2 is the last appeared coronavirus that developed a pandemic infection with huge number of fatal cases. No vaccines are yet available that protect from this infection. However, a number of therapeutic tactics against COVID-19 have been empirically started.
According to Mehra et al., 2020, in COVID-19 illness, a structured approach to clinical is imperative (1). Such approach distinguish the phase in which the viral pathogenicity is dominant, from the phase where the host inflammatory response overtakes the pathology. Therefore, after five months of epidemy, 3-stage’s progression of illness, corresponding to increasing severity with distinct clinical signs, therapy responses and clinical outcomes, has been assessed. These stages constitute the reference for investigation and proposition of effective targeted therapies (1).
Stage I is the phase where the early infection causes to most people mild or asymptomatic disease. Treatment at this level with drug having antiviral activity could prevent the progression to severe disease. Stage II (moderate) corresponds to the pulmonary involvement without (IIa) and with (IIb) hypoxia. In this stage the pulmonary disease is established with viral lung multiplication and localized inflammation. The patients develop a viral pneumonia, with cough, fever and possibly hypoxia. Now, not only antiviral drugs are required but, in particular, the inflammation has to be considered and treated. Stage III is the phase in which an extra-pulmonary systemic hyperinflammation syndrome manifests. Likely, a minority of Covid-19 patients transit to this stage where the therapy is essentially against the so called “inflammation storm… Continue reading.
UC Davis Engineering Projects Fight COVID-19
Cristina Davis | Via UC Davis | May 20, 2020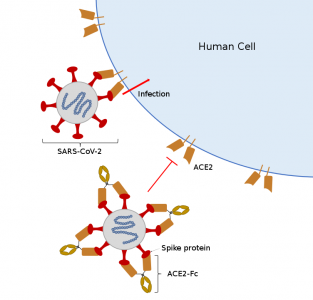
SARS-COV-2, also known as COVID-19, uses its spike proteins to bind with cells. Using antibodies or an enzyme like ACE2-FC to bind with the spike proteins is key to preventing further infection. Graphic courtesy of Roland Faller.
With new seed grants from the UC Davis Office of Research’s COVID-19 Research Accelerator Funding Track (CRAFT), three teams of UC Davis engineers are applying their expertise toward the pandemic response to help people become safer, healthier and better-tested.
Mechanical and aerospace engineering (MAE) professor and chair Cristina Davis and chemical engineering (CHE) faculty Priya Shah, Karen McDonald and Roland Faller received $25,000 project awards for research that rapidly generates new insights about COVID-19, while biological and agricultural engineering (BAE) professor Gang Sun received a $5,000 small award to apply current research to the pandemic response. These proposals were chosen out from more than 100 applications and were awarded with the expectation that these projects will lead to larger collaborations.
Using Breath to Predict COVID-19 Cases
MAE professor Cristina Davis and her team are using portable devices that capture people’s breath to look for chemical and biological markers, known as biomarkers, to identify and predict the severity of COVID-19 infections.
Davis’ lab produces portable chemical sensing devices that record chemicals from the air or a person’s breath using mass spectroscopy. As part of an ongoing project with professor Nick Kenyon and associate professor Michael Schivo at the UC Davis Medical Center, the team had been conducting a clinical study with these devices to try to find early, asymptomatic biomarkers for the flu. When the pandemic began, the shift to COVID-19 was an obvious one… Continue reading.
Researchers Receives NIH Funds for Adjuvant Research to Boost Coronavirus Vaccines
Krishnendu Roy | Via Georgia Tech | May 20, 2020Researchers have received funding from the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, to screen and evaluate certain molecules known as adjuvants that may improve the ability of coronavirus vaccines to stimulate the immune system and generate appropriate responses necessary to protect the general population against the virus.
“The adjuvants that we are studying, known as pathogen-associated molecular patterns (PAMPs), are molecules often found in viruses and bacteria, and can efficiently stimulate our immune system,” explained Krishnendu Roy, a professor and Robert A. Milton Chair in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University. “Most viruses have several of these molecules in them, and we are trying to mimic that multi-adjuvant structure… Continue reading.
Johns Hopkins researchers to use machine learning to predict heart damage in COVID-19 victims
Natalia Trayanova | Via Johns Hopkins University | May 18, 2020Johns Hopkins researchers recently received a $195,000 Rapid Response Research grant from the National Science Foundation to, using machine learning, identify which COVID-19 patients are at risk of adverse cardiac events such as heart failure, sustained abnormal heartbeats, heart attacks, cardiogenic shock and death.
Increasing evidence of COVID-19’s negative impacts on the cardiovascular system highlights a great need for identifying COVID-19 patients at risk for heart problems, the researchers say. However, no such predictive capabilities currently exist.
“This project will provide clinicians with early warning signs and ensure that resources are allocated to patients with the greatest need,” says Natalia Trayanova, the Murray B. Sachs Professor in the Department of Biomedical Engineering at The Johns Hopkins University Schools of Engineering and Medicine and the project’s principal investigator… Continue reading.
Carlsbad biotech to test drug that targets gut in COVID-19 patients
Geert Schmid-Schönbein | Via San Diego Union Tribune | May 15, 2020Leading Biosciences hopes that its drug will keep digestive enzymes from spilling into the bloodstream and triggering the airway inflammation seen in COVID-19
 COVID-19’s worst symptoms are felt in the lungs — where the airways of some patients fill with dead cells and fluid, triggering a deadly spiral of inflammation. A local biotech company thinks it can treat these symptoms by targeting a completely different part of the body: your gut.
COVID-19’s worst symptoms are felt in the lungs — where the airways of some patients fill with dead cells and fluid, triggering a deadly spiral of inflammation. A local biotech company thinks it can treat these symptoms by targeting a completely different part of the body: your gut.
Leading Biosciences received permission from the Food and Drug Administration on Friday to test whether its drug, designed to shore up the intestine’s natural barrier, helps severe COVID-19 patients breathe on their own and recover sooner.
It’s an unorthodox-sounding approach, but it’s based on the idea that many diseases — including COVID-19 — are connected to what happens in the gut… Continue reading.
Coventor-A COVID-19 Ventilation System
Arthur Erdman | Via Earl E. Bakken MDC | May 12, 2020In response to COVID-19, the Earl E. Bakken Medical Devices Center has built a homemade ventilator. This device represents a rapidly scalable opportunity for healthcare providers to provide life sustaining mechanical ventilation to patients for whom no other option currently exists. The mechanical ventilator is simple to use for ICU-trained medical providers, it is compact (the size of a cereal box) and relatively inexpensive to manufacture and distribute. This device does not require a pressurized oxygen or air supply unlike commercially available mechanical ventilators.
In collaboration between the University of Minnesota, local industry leaders, and individual donors, we are able to acquire the necessary components for prototypes required to test and prove the concept. The team is hoping to ramp up and provide enough ventilators to help alleviate the stress on the healthcare system in the wake of COVID-19. All ventilators will currently ship from Minneapolis, Minnesota.
Your gift will benefit the Earl E. Bakken Medical Devices Center educational, research, and outreach programs, with the main priority right now being the COVID-19 ventilator project.
Northeastern Professor Partners With Audax Medical Inc., to Combat Covid-19
Tom Webster | Via Northeastern University | May 12, 2020The Problem At Hand
The COVID-19 pandemic has changed many aspects of daily modern life; employees work from home, students attend class online, and individuals have been encouraged to stay inside, only leaving isolation for the essentials. The response has provided a mild reprieve from the rapid spread of the virus, though it is a temporary solution to a problem without a clear end in sight. Companies, universities and research labs involved in the health sciences across the world have shifted their focuses to combating COVID-19 and viral outbreaks to provide a better, more permanent solution for overcoming pandemic.
Recently, alongside Stanford University, Harvard University, and the Massachusetts Institute for Technology, the Center for Research Innovation at Northeastern University announced their support of the COVID-19 Technology Access Framework, which is a set of licensing principles that aims to make technologies that could aid research in preventing, diagnosing and treating COVID-19 more available. We took a moment to talk to Thomas Webster, a faculty researcher at Northeastern, about his work on one of these technologies.
The Inventor
Webster is a Professor at the College of Engineering at Northeastern University and heads a research lab responsible for researching and developing advanced nano-molecular technology that he calls the “Nano-Medicine Lab.” Earlier this month, Audax Medical, Inc., a Massachusetts-based company dedicated to developing medical innovations, licensed a technology developed in Webster’s lab that utilizes a nano-molecular approach to viral therapy… Continue reading.
Alternative to the Handshake Developed by UConn Doctors
Cato Laurencin | Via UConn Today | May 12, 2020 Did you know a single handshake can transfer 124 million bacteria?
Did you know a single handshake can transfer 124 million bacteria?
That’s why in the midst of the global COVID-19 pandemic in the journal Science’s Editor’s Blog entitled “The end of the handshake?,” UConn Health doctors are recommending a new alternative to the handshake to reduce human contact, protect public health, and diminish the spread of the coronavirus.
With hand-to-hand contact now strongly discouraged, and even the popular elbow bump now considered a breeding ground for germs due to the common practice of sneezing and coughing into the elbow region, Dr. Cato T. Laurencin and Dr. Aneesah McClinton of UConn Health’s Connecticut Convergence Institute for Translation in Regenerative Engineering have created “The Laurencin-McClinton Greeting” (LMG) to meet the evolving COVID-19 culture needs… Continue reading.
What they’re trained for
Paul Dayton | Via NC State University | May 11, 2020Biomedical engineers at UNC-Chapel Hill and NC State respond to COVID-19 by teaming to speed the development of an emergency ventilator
A prototype of the CaRE-Vent emergency ventilator, which can be
manufactured with six hours of skilled labor for less than $1,000 per unit
(Photo by UNC-Chapel Hill)
Biomedical engineering student Kathlyne Bautista always knew that her coursework and training would set her on a path to make a life-changing difference for people. But before the coronavirus pandemic, she didn’t realize just how soon that opportunity would arrive.
Bautista is part of the Carolina Respiratory Emergency – Ventilator (CaRE-Vent) team led by Dr. Yueh Lee, MD, an associate professor at the University of North Carolina at Chapel Hill. His research team is sprinting to design and prototype an open-source ventilator in a matter of weeks that has the potential to help fill a critical equipment gap caused by a projected spike in COVID-19 patients. The group is designing the ventilator so that it could be manufactured quickly and inexpensively – at less than $1,000 and with only six hours of skilled labor per unit.
And even in the best-case scenario – where the COVID-19 curve flattens to the point that the device is never needed for patients – the team’s efforts are advancing knowledge in the biomedical design community about the best way to create emergency ventilators in the future… Continue reading.
COVID-19 Puts Spotlight on Artificial Intelligence
Russ Altman | Via Genetic Engineering & Biotechnology News | May 11, 2020As the COVID-19 pandemic continues to infect people across the world, a technological application already familiar to many in the biotech field is lending a key supporting role in the fight to treat and stop it: artificial intelligence (AI).
AI is currently being used by many companies to identify and screen existing drugs that could be repurposed to treat COVID-19, aid clinical trials, sift through trial data, and scour through patient electronic medical records (EMRs). The power of AI in COVID-19 is that it is being used to generate actionable information—some of which would be impossible without AI—much more quickly than before.
A simple definition of AI is the ability of a computer to rapidly think and learn. AI utilizes machine learning to analyze large amounts of data. It can also model predictions, screen virtually and develop insights that can be used to advance R&D and make patient medical assessments… Continue reading.
Researchers to develop AI to help diagnose, understand COVID-19 in lung images
Maryellen Giger | Via University of Chicago | May 6, 2020UChicago, Argonne study hopes to learn to identify cases and guide treatment
As physicians and researchers grapple with a rapidly-spreading, deadly and novel disease, they need all the help they can get. Many centers are exploring whether artificial intelligence can help fight COVID-19, extracting knowledge from complex and rapidly growing data on how to best diagnose and treat patients.
One University of Chicago and Argonne National Laboratory collaboration believes that AI can be a helpful clinical partner for a particularly important kind of medical data: images. Because severe cases of COVID-19 most often present as a respiratory illness, triggering severe pneumonia in patients, chest X-rays and thoracic CT scans are a potential exam. With a grant from the new c3.ai Digital Transformation Institute, computer-aided diagnosis expert Maryellen Giger will lead an effort to develop new AI tools that use these medical images to diagnose, monitor and help plan treatment for COVID-19 patients… Continue reading.
Carnegie Mellon, Pitt Researchers Launch Ventilator Project
Keith Cook | Via Carnegie Mellon University | May 6, 2020Low-cost device could address current and future shortages
Researchers at Carnegie Mellon University and the University of Pittsburgh School of Medicine are developing a new, low-cost ventilator they say will address the ventilator shortage, both now and in the future, that has been made evident by the COVID-19 pandemic.
Dubbed Roboventilator, the device will employ CMU-developed robotic technologies and advanced sensors, filling the gap between the expensive sophisticated mechanical ventilators used in intensive care units and the current low-cost alternatives with limited capabilities being approved emergently by the Food and Drug Administration.
“We’ve already developed robotic and sensor technology that can detect force even as it drives an air pump,” said Howie Choset, professor of robotics at CMU. “When that is paired with air-management controls developed by Keith Cook, a CMU professor of biomedical engineering, we believe we can build a closed-loop system that can provide customized and appropriate ventilation to people with respiratory failure from COVID-19… Continue reading.
The Covid Recovery Comes Down to Engineering
Guru Madhavan | Via Wall Street Journal | May 5, 2020Don’t believe it? Look at the logistics required for the broadband everyone teleworking enjoys.
Reopening the country in the midst of a pandemic is akin to charging an enemy position at the top of a hill. Recovery and rebuilding will test us at every step with the risk of losing hard-won ground. But an old military insight can provide us with surer footing: amateurs talk tactics, professionals discuss logistics. Epidemiology established the right methods for fighting individual battles with Covid-19, from hand-washing to social distancing, but now it’s time to talk recovery logistics.
Much of the conversation will come back to engineering, which historically has advanced public health far more than medical care has. Sanitation, water supply, electrification, refrigeration,
highways, transportation safety, body scanning and mass production are a few examples. It’s easy to overlook how these technologies improve health outcomes, so consider one that’s an obvious part of many Americans’ lives today: the bandwidth necessary for telework… Continue reading.
ALung Announces Commercial Development of its Breakthrough Next Generation Artificial Lung
William Federspiel | Via Business Wire | May 5, 2020 ALung Technologies, Inc., the leading provider of low-flow extracorporeal carbon dioxide removal (ECCO2R) technologies for treating patients with acute respiratory failure, announced the recent initiation of commercial development of its next generation artificial lung, which expands the Company’s focus on highly efficient gas exchange devices and also broadens its applicable market.
ALung Technologies, Inc., the leading provider of low-flow extracorporeal carbon dioxide removal (ECCO2R) technologies for treating patients with acute respiratory failure, announced the recent initiation of commercial development of its next generation artificial lung, which expands the Company’s focus on highly efficient gas exchange devices and also broadens its applicable market.
The Company’s current product, the Hemolung® Respiratory Assist System (RAS), is the only fully comprehensive extracorporeal carbon dioxide removal (ECCO2R) system specifically designed and manufactured for this therapy, as compared to complex competitive products that are modifications of existing technologies designed for other purposes. The Hemolung continues to be the most highly efficient and simple to use ECCO2R system on the market today… Continue reading.
Monitoring COVID-19 from Hospital to Home: First Wearable Device Continuously Tracks Key Symptoms
John Rogers | Via Northwestern University | May 4, 2020Wireless sensor gently sits on throat to monitor coughs, fever, respiratory activity
The more we learn about the novel coronavirus (COVID-19), the more unknowns seem to arise. These ever-emerging mysteries highlight the desperate need for more data to help researchers and physicians better understand — and treat — the extremely contagious and deadly disease.
Researchers at Northwestern University and Shirley Ryan AbilityLab in Chicago have developed a novel wearable device and are creating a set of data algorithms specifically tailored to catch early signs and symptoms associated with COVID-19 and to monitor patients as the illness progresses… Continue reading.
María José Alonso leads a USC project aimed at developing a new vaccine against COVID-19 based on mRNA
María José Alonso | Via MJ Alonso Lab | May 4, 2020Developing and evaluating in preclinical studies a new vaccine based on mRNA against SARS-CoV2 capable of inducing long-term immune responses against the virus is the ultimate goal of the research project in which the laboratory led by María José Alonso participates together with the group led by Mabel Loza, both at CiMUS and FIDIS – University of Santiago de Compostela (USC). The objective of the USC laboratories is to produce a synthetic vehicle based on innocuous biomaterials, capable of transporting the mRNA into the target cells and enabling the production of the antigen in the human body.
The project has obtained funding from the Health Department of the Generalitat de Catalunya and the Carlos III Health Institute (ISCIII)… Continue reading.
NLM Highlights Essential Role of Clinical Databases in Pandemic
Patricia Brennan | Via GovernmentCIO Media | May 4, 2020The National Library of Medicine is embarking on an extensive modernization effort of the world’s largest public clinical trial registry and results database, ClinicalTrials.gov, with the COVID-19 response underpinning the importance of the multi-year project.
“This effort to improve the user experience and update the technology platform is critically important for so many things that we do at NIH, our partnerships across the government and our commitment to the American public — the taxpayers and the research participants,” Kelly Wolinetz, associate director for the agency’s Office of Science Policy and NIH’s acting chief of staff, said in a virtual public meeting Thursday.
…
Additionally, although the novel coronavirus was discovered relatively recently — with the first full sequence of the virus published in NLM’s GenBank in mid-January — there are over 900 clinical trial studies for COVID-19 listed on the website, NLM Director Patricia Brennan noted.
“During this crisis, many libraries have closed and over the last five years many hospital libraries have restricted their services, so never before has the NLM been this important… Continue reading.
Protecting the Heart from COVID-19
Todd McDevitt | Via Gladstone Institutes | May 1, 2020 It’s well-known that COVID-19 affects the respiratory system, infecting healthy lung cells with the COVID-19 virus, but if it spreads to the heart it could become a much more deadly disease. A recent study found that in more than 10 percent of COVID-19 cases where heart damage occurred, there was no history of cardiovascular disease. Furthermore, a blood marker for heart damage (troponin) was the single best predictor of death, suggesting that heart damage is a key factor in mortality. Now the virus has been found in heart tissue, and the virus can infect human heart cells in a dish, stopping them from beating. Investigating the link between COVID-19 and damage to the heart is vital to preventing cardiovascular effects in future patients and perhaps finding a treatment for COVID-19 induced heart failure.
It’s well-known that COVID-19 affects the respiratory system, infecting healthy lung cells with the COVID-19 virus, but if it spreads to the heart it could become a much more deadly disease. A recent study found that in more than 10 percent of COVID-19 cases where heart damage occurred, there was no history of cardiovascular disease. Furthermore, a blood marker for heart damage (troponin) was the single best predictor of death, suggesting that heart damage is a key factor in mortality. Now the virus has been found in heart tissue, and the virus can infect human heart cells in a dish, stopping them from beating. Investigating the link between COVID-19 and damage to the heart is vital to preventing cardiovascular effects in future patients and perhaps finding a treatment for COVID-19 induced heart failure.
Senior Investigators Bruce Conklin, MD, and Todd McDevitt, PhD, are investigating how COVID-19 might damage the heart by asking two questions: How susceptible are the cells in the heart to infection by the virus, and what pharmaceuticals could be used to lessen damage to the heart or prevent the virus from infecting heart cells altogether… Continue reading.
Cellular & Molecular Bioengineering Special Issue on ‘Emerging Technologies for Use in the Study, Diagnosis and Treatment of Patients with COVID-19’
Michael King | Via CAMB | April 30, 2020COVID-19 is predicted to overwhelm healthcare capacity in the US and worldwide. The cellular and molecular bioengineering community has a history of innovative approaches to address pressing biomedical challenges. As a voice for the this community, the journal Cellular & Molecular Bioengineering (CAMB) welcomes commentaries, reviews, and original research articles that reflect the ways in which we continue to contribute to fields that have become central to understanding, treating, and managing the COVID-19 pandemic. Some examples include:
- On-a-chip platforms for diagnostics, drug screening, toxicology, and basic research;
- Innovative approaches to reduce cytokine “storms”;
- Technologies to understand, diagnose, and treat cardiovascular and other COVID-19 comorbidities;
- Understanding sensory function of taste and smell;
- Platforms to understand, diagnose, and treat zoonoses;
- Commentary from trainees, faculty, and administrators.
A team of physician-scientists have volunteered to help refine and provide constructive feedback to encourage contributors who may not be content experts in virology or patient care. These articles will be free for download at CAMB thanks to the generous support of publisher Springer Nature… Download the full document.
BrunO2 project, Tripathi Lab earn University COVID-19 Research Seed Fund awards
Anubhav Tripathi | Via Brown University | April 30, 2020Brown Engineering professors Dan Harris, Jacob Rosenstein, Anubhav Tripathi and Roberto Zenit, are among the 15 teams of Brown faculty researchers receiving funds from a newly created University seed fund. Brown established the fund to fast track innovative research proposals that directly address the urgent needs of the COVID-19 pandemic. A total of $350,000 was awarded to support research with the potential for significant and rapid impact on human health and research that could create products of immediate need for the healthcare system in Rhode Island and the nation.
…
Tripathi is teaming with Alpert Medical School professor and infectious disease expert Rami Kantor, M.D., to develop a molecular surveillance tool and capacity to monitor spread of the virus regionally and beyond. Its goal is understanding if, and how, genotypic variants of SARS-CoV-2 might impact patient outcome; the investigation of the viral mutability under treatment selection pressure; and transmission dynamics of the virus in Rhode Island and beyond, informing public health interventions… Continue reading.
OHIO researchers win grant to study treatment for possible fatal complications of COVID-19
Doug Goetz | Via Ohio University | April 30, 2020Faculty researchers from Ohio University’s Heritage College of Osteopathic Medicine and Russ College of Engineering and Technology have received a $100,000 grant to investigate possible treatments for mitigating the severity of COVID-19.
Kelly McCall, Ph.D., and Douglas Goetz, Ph.D., will measure how effective a number of different chemical compounds are at preventing “cytokine storms,” a sometimes-fatal complication that can stem from COVID-19 infections.
The body responds to the presence of a pathogen by releasing a swarm of immune system proteins called cytokines to help fight off the virus or bacterium. If too many cytokines are released, a cytokine storm develops which can severely damage organs. This reaction is believed to be responsible for some of the deaths from COVID-19… Continue reading.
UC Davis Engineers, Clinician Develop Low-Cost, Portable Ventilator
Tingrui Pan | Via UC Davis | April 29, 2020Engineers at the University of California, Davis, are working with clinicians to create a simple, inexpensive ventilator. They have developed a prototype and plan to make plans freely available online. Versions could be in clinical use in about six months. “This is a critical device to have. It provides the vital functions of a ventilator while being completely portable,” said Andrew Li, assistant professor in the Department of Surgery at UC Davis Health. The device, named “AmbuBox,” is based on the ambu-bag, a handheld ventilation device. Squeezing the bag by hand pushes air into a patient’s lungs.
There is a gap between the handheld ambu-bag and expensive ventilators used in intensive care units, said Tingrui Pan, professor of biomedical engineering at UC Davis. One attempt to fill this gap is MIT’s E-VENT project, but this device involves a number of mechanical moving parts… Continue reading.
Giving Distressed Lungs a Safer Fighting Chance
William Federspiel | Via Global Health News wire | April 28, 2020A device designed at the University of Pittsburgh could help improve outcomes as a treatment for COVID-19 when used in conjunction with non-invasive or mechanical ventilation, and it recently received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration. Health records from a New York study showed that close to 90 percent of patients who were placed on mechanical ventilation did not survive. Some intensive care units are now considering mechanical ventilation as a last resort because of the complications and side effects associated with the process, and researchers believe this device could help.
The Hemolung Respiratory Assist System is a minimally invasive device that does the work of the lungs by removing carbon dioxide directly from the blood, much as a dialysis machine does the work of the kidneys. The device was developed by William Federspiel, PhD, professor of bioengineering at Pitt’s Swanson School of Engineering, and the Pittsburgh-based lung-assist device company ALung Technologies, co-founded by Federspiel… Continue reading.
Portable Microfluidic Platform Developed for Detecting Coronavirus Using Smartphone
Rashid Bashir | Via Genetic Engineering & Biotechnology News | April 24, 2020Researchers headed by a team at the University of Illinois, Urbana-Champaign, have developed what they claim is an inexpensive, sensitive smartphone-based device that can detect viral and bacterial pathogens in about 30 minutes, and could be adapted to test for SARS-CoV-2. The platform comprises a cartridge-housed microfluidic chip that carries out isothermal amplification of viral nucleic acids from nasal swab samples, which are then detected using the smartphone camera. The investigators report on their use of the system to detect equine viruses as a non-biohazard surrogate for SARS-CoV-2, but say that when adapted to test for coronavirus, the smartphone accessory, costing about $50, could be used to reduce the pressure on testing laboratories during pandemics such as COVID-19.
“This test can be performed rapidly on passengers before getting on a flight, on people going to a theme park, or before events like a conference or concert,” said University of Illinois, Urbana-Champaign electrical and computer engineering professor Brian Cunningham, PhD, who, together with bioengineering professor Rashid Bashir, PhD, led the development of the device. “Cloud computing via a smartphone application could allow a negative test result to be registered with event organizers or as part of a boarding pass for a flight. Or, a person in quarantine could give themselves daily tests, register the results with a doctor, and then know when it’s safe to come out and rejoin society… Continue reading.
Portable Microfluidic Platform Developed for Detecting Coronavirus Using Smartphone
Brian Cunningham | Via Genetic Engineering & Biotechnology News | April 24, 2020Researchers headed by a team at the University of Illinois, Urbana-Champaign, have developed what they claim is an inexpensive, sensitive smartphone-based device that can detect viral and bacterial pathogens in about 30 minutes, and could be adapted to test for SARS-CoV-2. The platform comprises a cartridge-housed microfluidic chip that carries out isothermal amplification of viral nucleic acids from nasal swab samples, which are then detected using the smartphone camera. The investigators report on their use of the system to detect equine viruses as a non-biohazard surrogate for SARS-CoV-2, but say that when adapted to test for coronavirus, the smartphone accessory, costing about $50, could be used to reduce the pressure on testing laboratories during pandemics such as COVID-19.
“This test can be performed rapidly on passengers before getting on a flight, on people going to a theme park, or before events like a conference or concert,” said University of Illinois, Urbana-Champaign electrical and computer engineering professor Brian Cunningham, PhD, who, together with bioengineering professor Rashid Bashir, PhD, led the development of the device. “Cloud computing via a smartphone application could allow a negative test result to be registered with event organizers or as part of a boarding pass for a flight. Or, a person in quarantine could give themselves daily tests, register the results with a doctor, and then know when it’s safe to come out and rejoin society… Continue reading.
Unique System for Using UVC Light to Sterilize Masks in Bulk Developed at Rensselaer
Deepak Vashishth | Via Rensselaer Polytechnic Institute | April 23, 2020New device for making masks reusable in fight against COVID-19 being tested at Mount Sinai
The shortage of critical personal protective equipment (PPE) has been a persistent problem for medical and other front-line workers as they battle the COVID-19 pandemic at close range day after day. A team of researchers at Rensselaer Polytechnic Institute has developed a potential solution: a machine that uses ultraviolet (UVC) light to sterilize thousands of protective masks each day, rendering them safe for reuse.
“At Rensselaer, we are focused on solving global challenges, and the COVID-19 pandemic is certainly among the most significant of these in our lifetimes,” said Rensselaer President Shirley Ann Jackson. “Our interdisciplinary approach, combined with the passion and ingenuity of our researchers, enables us to contribute solutions in this crisis that will continue to be helpful even after it has passed… Continue reading.
One-step diagnostic tool receives NSF RAPID grant
Michael Jewett | Via Northwestern University | April 20, 2020Northwestern University synthetic biologists have received funding to develop an easy-to-use, quick-screen technology that can test for infectious diseases, including COVID-19, in the human body or within the environment.
Similar to a pregnancy test, the tool uses one sample to provide an easy-to-read negative or positive result. By simplifying testing, the researchers could put diagnostics into the hands of people everywhere — without the need for expensive laboratories or expertise. This could provide the large-scale testing required for ending stay-at-home orders, reopening the economy or preparing for a predicted virus resurgence in the fall… Continue reading.
4 Ways Engineers Aim to Save Lives During COVID-19
Mehmet Toner | Via Futurity | April 20, 2020Researchers are pivoting their work to tackle the many engineering problems associated with the global coronavirus pandemic.
…
2. A NEW, FASTER COVID-19 TEST
Researchers are working to develop a rapid and reliable test for the SARS-CoV-2 virus.
The currently available tests look for the presence of SARS-CoV-2’s viral RNA, a unique and identifying genetic code. Building on previous research, the new test is fundamentally different: it detects and counts individual SARS-CoV-2 viruses by capturing them with antibodies.
The primary benefit of this approach is that its testing mechanism doesn’t require extensive sample preparation.
It also reduces the chance of false negative results. Viruses can mutate, but the currently available tests rely on knowing specific genetic sequences of the virus to detect it. So, if the coronavirus mutates within one of those sequences, a current test could report a false negative — which happened during the 2014 Ebola outbreak, making it difficult to accurately diagnose who was sick and contain the outbreak.
The new test uses a different set of supplies than the existing test, leaving it less prone to supply chain shortages than the current method, says Selim Ünlü, a professor of electrical, computer, materials science, and biomedical engineering, who is teaming up with John Connor, associate professor of microbiology at the School of Medicine, from the National Emerging Infectious Diseases Laboratories, and Mehmet Toner of Massachusetts General Hospital to develop the test… Read the full article.
BU Engineers Are Taking on the Coronavirus Pandemic
Joyce Wong | Via Boston University | April 17, 2020Across Boston University’s School of Engineering, researchers are pivoting their work to tackle the many engineering problems associated with the global coronavirus pandemic.
“I’m glad I’m an engineer right now,” says Joyce Wong, professor of biomedical and materials science engineering. “There are so many problems that need to be solved in this crisis and I can actually use my expertise to help.”
Wong, like many other engineers and researchers, is diving in to do what she can to mitigate the COVID-19 pandemic. These efforts are in addition to the first wave of help, across BU’s Charles River and Medical Campuses, that gathered personal protective equipment (PPE) from labs—shuttered by Governor Charlie Baker’s stay-at-home advisory—to donate to healthcare workers in Massachusetts. Here are four ways that BU engineers are using technology to tackle the coronavirus pandemic:
1. New medical equipment
Wong started working on two projects after talking to her cousin, Steven Horng, an emergency medicine physician at Beth Israel Deaconess Medical Center (BIDMC).
“I started hearing about the PPE shortages from Steven, and then he started to tell me about more of the challenges healthcare workers are facing,” she says. “We’re getting close to the predicted peak of cases in Massachusetts, so I want to help out any way I can… Continue reading.
BU Engineers Are Taking on the Coronavirus Pandemic
Catherine Klapperich | Via Boston University | April 17, 2020Across Boston University’s School of Engineering, researchers are pivoting their work to tackle the many engineering problems associated with the global coronavirus pandemic.
…
3. Speeding up test validation
Catherine Klapperich, director of the BU Precision Diagnostics Center and a professor of biomedical and materials science engineering, is spearheading a team to validate new types of SARS-CoV-2 tests. To contain the current COVID-19 pandemic, and prevent future relapses, an extreme ramp up of testing is needed across the United States. But there are currently roadblocks and shortages of supplies barring that from being possible. To increase testing capabilities, new tests, like Ünlü’s, must be evaluated and validated through FDA regulatory procedures. Those validations take time—so, Klapperich’s team is trying to speed up that process.
The Precision Diagnostics Center is taking on the task of preclinical lab validation of newly developed COVID-19 tests. First up, they’re working with one developed by Michael Springer’s systems biology group at Harvard Medical School… Continue reading.
BU Engineers Are Taking on the Coronavirus Pandemic
Selim Ünlü | Via Boston University | April 17, 2020Across Boston University’s School of Engineering, researchers are pivoting their work to tackle the many engineering problems associated with the global coronavirus pandemic.
…
2. A novel (and more rapid) COVID-19 test
Selim Ünlü, BU professor of electrical, computer, materials science and biomedical engineering, is teaming up with longtime collaborator John Connor, BU School of Medicine associate professor of microbiology, from the National Emerging Infectious Diseases Laboratories. Together with Mehmet Toner of Massachusetts General Hospital, the trio is working to develop a rapid and reliable test for the SARS-CoV-2 virus.
The currently available tests look for the presence of SARS-CoV-2’s viral RNA, a unique and identifying genetic code. Building on his previous research, Ünlü’s test is fundamentally different: it detects and counts individual SARS-CoV-2 viruses by capturing them with antibodies… Continue reading.
3D printed swabs developed at UofL to help fill gap in COVID-19 test kits
George Pantalos | Via University of Louisville | April 16, 2020 Innovation at the University of Louisville involving multiple departments at the university has led to a promising solution for the shortage of swabs in COVID-19 test kits. In response to a request from the Commonwealth of Kentucky, UofL’s Additive Manufacturing Institute of Science & Technology (AMIST), along with faculty and students in the Schools of Dentistry, Engineering and Medicine have created a 3D printed swab made of a pliable resin material.
Innovation at the University of Louisville involving multiple departments at the university has led to a promising solution for the shortage of swabs in COVID-19 test kits. In response to a request from the Commonwealth of Kentucky, UofL’s Additive Manufacturing Institute of Science & Technology (AMIST), along with faculty and students in the Schools of Dentistry, Engineering and Medicine have created a 3D printed swab made of a pliable resin material.
“This effort adds to the list of our response during the pandemic, including 3D printed face shields, respirators and ventilators being manufactured through the expertise of our institute. We hope our work will provide the necessary tools for Kentucky, as well as our local health care facilities,” said Ed Tackett, director of workforce development at AMIST, which is part of the J.B. Speed School of Engineering… Continue reading.
Vapor H2O2 sterilization as a decontamination method for the reuse of N95 respirators in the COVID-19 emergency
Ebru Oral | Via MedRxIV | April 16, 2020Abstract
There are a variety of methods routinely used in the sterilization of medical devices using hydrogen peroxide (H2O2) including vaporization, plasma generation and ionization. Many of these systems are used for sterilization and are validated for bioburden reduction using bacterial spores. Here, we explored the benefits of using vaporized H2O2 (VHP) treatment of N95 respirators for emergency decontamination and reuse to alleviate PPE shortages for healthcare workers in the COVID-19 emergency. The factors that are considered for the effective reuse of these respirators are the fit, the filter efficiency and the decontamination/disinfection level for SARS-CoV-2, which is the causative virus for COVID-19 and other organisms of concern in the hospital environment such as methicillin-resistant Staphylococcus aureus or Clostridium difficile. WE showed that the method did not affect fit or filter efficiency at least for one cycle and resulted in a >6 log reduction in bacterial spores and >3.8 log reduction in the infectious SARS-CoV2 load on N95 respirators… Read the full paper.
PPE Patent Pending: Next Generation Custom-fitting Masks
Cato Laurencin | Via UConn Today | April 16, 2020UConn is developing the latest innovative approach to tackling the personal protective equipment (PPE) shortage that has developed in the wake of the coronavirus pandemic, and it has already been used to protect front-line providers at UConn Health.
The Connecticut Convergence Institute for Translation in Regenerative Engineering has developed a method to fabricate custom-fit mask frames and exoskeletons to give conventional masks the optimal protective qualities of N95 respirators.
“We use a combination of facial recognition software and 3D printing to create the exact dimensions and make the perfect size,” says Dr. Cato T. Laurencin, the institute’s CEO. “It’s very difficult to make one-size fits all, and one size shouldn’t fit all… Continue reading.
Proteins may halt the severe cytokine storms seen in Covid-19 patients
Shuguang Zhang | Via MIT | April 16, 2020One of the defining features of Covid-19 is the excessive immune response that can occur in severe cases. This burst of immune overreaction, also called a cytokine storm, damages the lungs and can be fatal.
A team of MIT researchers has developed specialized proteins, similar in structure to antibodies, that they believe could soak up these excess cytokines.
“The idea is that they can be injected into the body and bind to the excessive cytokines as generated by the cytokine storm, removing the excessive cytokines and alleviating the symptoms from the infection,” says Rui Qing, an MIT research scientist who is one of the senior authors of the study… Continue reading.
Chafing Against Regulation, Silicon Valley Pivots to Pandemic
Michael Jewett | Via Wired | April 15, 2020Early in the evening on March 19, the prominent Silicon Valley investor and serial entrepreneur Balaji Srinivasan kicked off a tweet storm with a techno-libertarian call to arms:
“To all biotech & tech people: The Manhattan Project for the virus is going to end up being the Palo Alto Project. It’s on us. The state doesn’t have tech talent anymore. Can’t fix that overnight. But we can get them to legalize biomedical innovation with expanded right-to-try.”
Srinivasan did not respond to WIRED’S request for comment, but a subsequent tweet clarified that he was using the term Palo Alto Project to encapsulate the world of venture-backed “tech/biotech” companies that he envisions mobilizing to solve the mysteries of Covid-19 with the same awesome resolve with which J. Robert Oppenheimer and company cracked the atom… Continue reading.
Peptide Therapies Could Disable Coronavirus’ Spike Proteins
Sam Stupp | Via Northwestern University | April 13, 2020Nanostructures could safely deliver a notoriously fragile drug to virus
Researchers are developing new peptide-based therapeutics for targeting and disabling the coronavirus’ so-called “spike proteins.”
Spike proteins — the crown of bulbous projections that give the coronavirus its signature halo effect — attach to and infect healthy cells, causing COVID-19. Led by Northwestern University and Massachusetts Institute of Technology (MIT), the research team is engineering a new nanostructured therapy that could potentially disable the virus and prevent its infection of human cells.
The idea is based on a recent discovery from the laboratory of Bradley L. Pentelute, an associate professor of chemistry at MIT. Pentelute’s team discovered a peptide molecule that specifically and strongly binds to the coronavirus’ spike protein… Continue reading.
Prof. Odde developing a simulator to predict COVID-19 trial outcomes
David Odde | Via University of Minnesota | April 13, 2020Professor David Odde is creating a biophysical computer model that simulates COVID-19 on a molecular and cellular level, and tests therapies and vaccines computationally.
Researcher unraveling SARS-CoV-2 spike protein through music
Markus Buehler | Via Phys.org | April 10, 2020The proteins that make up all living things are alive with music. Just ask Markus Buehler: The musician and MIT professor develops artificial intelligence models to design new proteins, sometimes by translating them into sound. His goal is to create new biological materials for sustainable, non-toxic applications. In a project with the MIT-IBM Watson AI Lab, Buehler is searching for a protein to extend the shelf-life of perishable food. In a new study in Extreme Mechanics Letters, he and his colleagues offer a promising candidate: a silk protein made by honeybees for use in hive building.
In another recent study, in APL Bioengineering, he went a step further and used AI discover an entirely new protein. As both studies went to print, the Covid-19 outbreak was surging in the United States, and Buehler turned his attention to the spike protein of SARS-CoV-2, the appendage that makes the novel coronavirus so contagious. He and his colleagues are trying to unpack its vibrational properties through molecular-based sound spectra, which could hold one key to stopping the virus. Buehler recently sat down to discuss the art and science of his work… Continue reading.
UConn Researchers Find Blacks Are Disproportionately Impacted By COVID-19
Cato Laurencin | Via CT News Junkie | April 8, 2020The team led by Dr. Cato T. Laurencin, former dean of the UConn School of Medicine, analyzed and reviewed the Department of Public Health’s data on COVID-19 outcomes and found that Blacks have a higher rate of infection and death in comparison to the percentage of the population they represent in the state.
However, the information collected on race and ethnicity is incomplete.
“The scarcity of this information generates a more substantial concern in which insufficiently identifying the affected may ultimately result in historically marginalized groups shouldering the greatest burden of disease and disproportionately bearing the social impact,” Laurencin and his team wrote in their paper… Continue reading.
Vitamin D3 may reduce severity of COVID-19 respiratory viral infection
Raphael Lee | Via BMJ Publishing Group | April 8, 2020It is well established that the ubiquitously expressed master immune response transcription factor NF-κB is centrally involved in the pathogenesis of respiratory viral infections. [1] Indeed, COVID-19 is known to activate the NF-κB pathway that results in the upregulation of many inflammatory gene promoters. [2] It is postulated that this results from multiple catalytic interactions that occurs between the viral nucelocapsid proteins and NF-κB mediated immunomodulation. [3,4,5] This signaling dynamic is similar to the manner in which other highly fatal coronaviruses, such as MERS and SARS-COV, are known to take control of the NF-κB pathway. [6-8] The activation of the NF-kB pathway leads to the release of inflammatory mediators often linked to the systemic inflammatory response syndrome. [8]
Vitamin D3 (VD3) also is a regulator of NF-kB mediated cellular responses, It is known to inhibit the production of the proinflammatory cytokines TNFα, interleukins, and other key activators of the cellular immune defense. [9,10] VD3 promotes phenotypic shifting of lymphocytes towards an anti-inflammatory subsets. [11] Furthermore, data from clinical studies indicate that VD3 deficiency is clinically associated with increased susceptibility to respiratory viral infections and increased severity once infected. [12] In the case of respiratory syncytial virus, vitamin D3 increases synthesis of the NF-κB inhibitor, IκBα, resulting in decreased expression of pro-inflammatory genes… Continue reading.
UArizona professor submits designs for 3 low-cost ventilator prototypes to DOD
Marvin Slepian | Via KGUN9 | April 6, 2020The University of Arizona continues to contribute to the fight against COVID-19 with low-cost ventilator prototypes.
The team submitted three designs to the Department of Defense one using something nearly everyone has lying around, a basketball.
Director Uarizona center accelerated biomedical innovation Marvin Slepian, MD said “For the more seriously ill patient we needed to… Continue reading.
Racial Profiling Is a Public Health and Health Disparities Issue
Cato Laurencin | Via Springer Link | April 6, 2020Racial profiling is a public health and health disparities issue through its disparate and adverse health impact on those targeted by this practice, as well as members of their communities. We discuss six ways police profiling and racial discrimination adversely impact Black American health. We identify four direct and two indirect ways. Four direct ways are (1) violent confrontation with police that causes injury or death; (2) police language that escalates a confrontation through micro-aggressions or macro-aggressions; (3) sub-lethal confrontations with police; (4) adverse health consequences of perceived or vicarious threat, i.e., the mere belief in potential harm by police injures health. There are two indirect ways: (5) through knowledge of or personal relationship with someone who directly experienced racial profiling; (6) through public events without a personal knowledge of the unarmed person threatened or killed by police as a result of racial profiling, but where such events cause both individuals and the community at large to perceive a threat. We support recognition of racial profiling as a public health and health disparities issue. We recommend support for community programs that address the clinical health effects of racial profiling. We also recommend widespread engagement of trauma-informed policing (TIP) that acknowledges the clinical effects of racial profiling… Continue reading.
Cell-free Biotechnology Could Help Accelerate COVID-19 Therapeutics
Michael Jewett | Via Northwestern University | April 2, 2020When it comes to fighting a fast-spreading pandemic, speed is critical.
Researchers at Northwestern Engineering and Cornell University have developed a new platform that could produce new therapies more than 10 times faster than current methods. The secret behind the platform’s unmatched speed is an unlikely tool: bacteria.
After taking the molecular machinery out of bacteria, the researchers then use that machinery to make a product, such as therapeutics, in a safe, inexpensive, and rapid manner. The idea is akin to opening the hood of a car and removing the engine, which allows researchers to use the engine for different purposes, free from the constraints of the car… Continue reading.
MIT initiates mass manufacture of disposable face shields for Covid-19 response
Elazer Edelman | Via MIT | April 1, 2020The shortage of personal protective equipment (PPE) available to health care professionals has become increasingly problematic as Covid-19 cases continue to surge. The sheer volume of PPE needed to keep doctors, nurses, and their patients safe in this crisis is daunting — for example, tens of millions of disposable face shields will be needed nationwide each month. This week, a team from MIT launched mass manufacturing of a new technique to meet the high demand for disposable face shields.
The single piece face shield design will be made using a process known as die cutting. Machines will cut the design from thousands of flat sheets per hour. Once boxes of these flat sheets arrive at hospitals, health care professionals can quickly fold them into three-dimensional face shields before adjusting for their faces… Continue reading.
Robots to the Rescue: How They Can Help During Coronavirus (and Future Pandemics)
Guang-Zhong Yang | Via Singularity Hub | April 1, 2020As the coronavirus pandemic forces people to keep their distance, could this be robots‘ time to shine? A group of scientists think so, and they’re calling for robots to do the “dull, dirty, and dangerous jobs” of infectious disease management.
Social distancing has emerged as one of the most effective strategies for slowing the spread of COVID-19, but it’s also bringing many jobs to a standstill and severely restricting our daily lives. And unfortunately, the one group that can’t rely on its protective benefits are the medical and emergency services workers we’re relying on to save us.
Robots could be a solution, according to the editorial board of Science Robotics, by helping replace humans in a host of critical tasks, from disinfecting hospitals to collecting patient samples and automating lab tests… Continue reading.
Solving the Ventilator Shortage with Windshield Wiper Parts
Thomas Milner | Via The University of Texas at Austin | April 1, 2020Researchers at The University of Texas at Austin are building a new type of ventilator made of cheap, widely available materials to help fill the demand created by the spread of COVID-19 for these critical devices that help patients breathe.
Ventilators become necessary when patients can’t breathe on their own, physically pumping oxygen into their lungs. They are in short supply. That’s why the researchers are building a “bridge ventilator” that can be replicated and mass-produced by others… Continue reading.
Here’s How Nanoparticles Could Help Us Get Closer to a Treatment for COVID-19
Thomas Webster | Via Northeastern University | March 31, 2020There is no vaccine or specific treatment for COVID-19, the disease caused by the severe acute respiratory syndrome coronavirus 2, or SARS-CoV-2.
Since the outbreak began in late 2019, researchers have been racing to learn more about SARS-CoV-2, which is a strain from a family of viruses known as coronavirus for their crown-like shape.
Northeastern chemical engineer Thomas Webster, who specializes in developing nano-scale medicine and technology to treat diseases, is part of a contingency of scientists that are contributing ideas and technology to the Centers for Disease Control and Prevention to fight the COVID-19 outbreak… Continue reading.
Covid-19 diagnostic based on MIT technology might be tested on patient samples soon
Daniel Anderson | Via MIT | March 31, 2020As more Covid-19 cases appear in the United States and around the world, the need for fast, easy-to-use diagnostic tests is becoming ever more pressing. A startup company spun out from MIT is now working on a paper-based test that can deliver results in under half an hour, based on technology developed at MIT’s Institute for Medical Engineering and Science (IMES).
Cambridge-based E25Bio, which developed the test, is now preparing to submit it to the FDA for “emergency use authorization,” which would grant temporary approval for using the device on patient samples during public health emergencies… Continue reading.
Coronavirus Testing Shouldn’t Be This Complicated
Catherine Klapperich | Via The Verge | March 30, 2020Engineers have the technology to make it better
The US reported its first confirmed case of COVID-19 on January 21st. Eight weeks later, there still aren’t enough tests for the virus available for everyone who needs them. “It is a failing,” said Anthony Fauci, director of the National Institutes of Allergy and Infectious Diseases, at a House briefing last week. “The system is not really geared to what we need right now.”
People who are sick or have been in contact with sick people are struggling to get tested. Until last week, the number of tests that could be run per day in the United States was limited to around 7,000. Labs are struggling to get the supplies they need to meet the demand.
…
“If the health system is working well, those tests should be good and help us manage this epidemic,” says Catherine Klapperich, director of the Laboratory for Diagnostics and Global Healthcare Technologies at Boston University. “It’s frustrating that the testing we thought we could rely on didn’t roll out the way we expected it to… Continue reading.
Coronavirus Testing Shouldn’t Be This Complicated
Paul Yager | Via The Verge | March 30, 2020Engineers have the technology to make it better
The US reported its first confirmed case of COVID-19 on January 21st. Eight weeks later, there still aren’t enough tests for the virus available for everyone who needs them. “It is a failing,” said Anthony Fauci, director of the National Institutes of Allergy and Infectious Diseases, at a House briefing last week. “The system is not really geared to what we need right now.”
People who are sick or have been in contact with sick people are struggling to get tested. Until last week, the number of tests that could be run per day in the United States was limited to around 7,000. Labs are struggling to get the supplies they need to meet the demand.
…
PCR is the gold-standard testing platform for viruses because it’s highly sensitive, says Paul Yager, a professor in the department of bioengineering at the University of Washington — it can detect even a tiny amount of virus in a patient sample and is less likely to incorrectly have a negative result… Continue reading.
Futuristic Technology Prints 1,000 Face Shield Components Per Day
Chad Mirkin | Via Northwestern University | March 30, 2020Researchers hope to make a dent in hospitals’ need with a single 3D printer
Northwestern University researchers have demonstrated the ability to generate 1,000 components for face shields per day — with a single 3D printer.
A critical piece of personal protective equipment (PPE), face shields protect health care workers from the novel coronavirus (COVID-19) as they treat patients.
When Northwestern researchers Chad A. Mirkin and David Walker heard about the PPE shortage in hospitals, their team sprang into action. In October, Mirkin and his research group, in a breakthrough article in the journal Science, unveiled a new 3D printing technique called “high-area rapid printing” (HARP), a 13-feet-tall printer with a 2.5 square-foot print bed that can print about half a yard in an hour — a record throughput for the 3D printing field… Continue reading.
Open Source Ventilator Project
Samsun Lampotang | Via University of Florida | March 28, 2020Open Source, Open Architecture Ventilator Engineering Design Specifications
This open source project has been created to address predicted ventilator shortage worldwide due to the COVID-19 pandemic and host open source contributions – Click here for details.
How Does a Library Respond to a Global Health Crisis?
Patricia Brennan | Via National Library of Medicine | March 24, 2020Around the world, scientists, public health officials, medical professionals, and others are working to address the coronavirus pandemic.
At NLM, we’ve been working on multiple fronts to improve researchers’ understanding of SARS-CoV-2 (the virus that causes the novel coronavirus) and aid in the response to COVID-19 (the disease caused by the novel coronavirus). By enhancing access to relevant data and information, NLM is demonstrating how libraries can contribute in real time to research and response efforts during this crisis… Continue reading.
 AIMBE
AIMBE